Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is associated with an increased risk of lung cancer compared to the general population. While smoking is a shared risk factor, little is known about the association between use of disease modifying anti-rheumatic drugs (DMARDs) and development of lung cancer. We undertook this study to evaluate the association between use of conventional synthetic (cs) versus biologic (b) or targeted synthetic (ts) DMARDs and incident lung cancer in patients with RA.
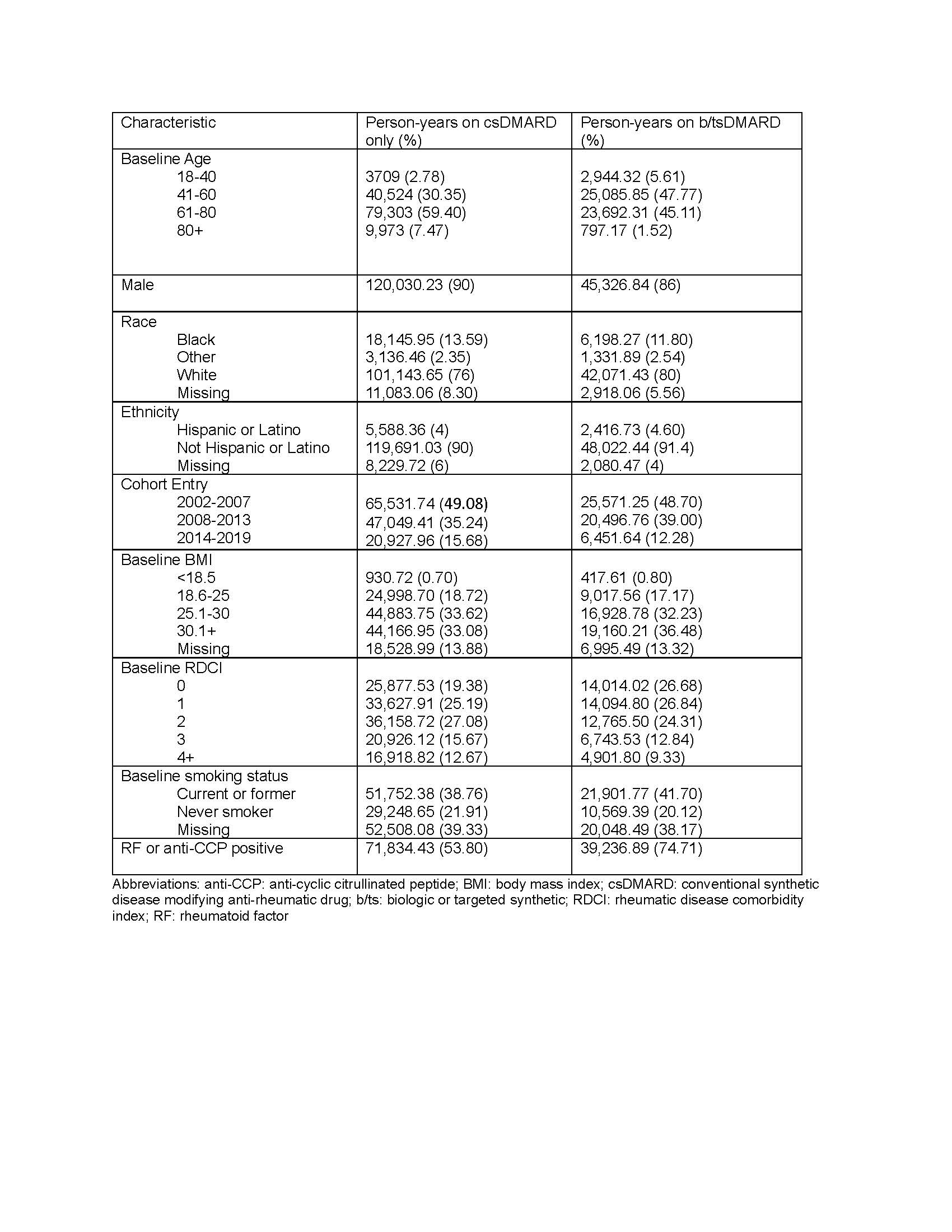
Methods: In this retrospective cohort study, we identified Veterans diagnosed with RA in any United States Veterans Affairs facility from 1/1/2002 and 12/31/2018. Criteria for the presence of RA were: two or more International Classification of Diseases Version 9 or 10 (ICD9 or ICD10) diagnosis codes for RA at least 7 days apart but no more than 365 days apart, and a prescription for a csDMARD within 90 days of the first RA diagnosis (index date). The csDMARDs included in these analyses were: methotrexate, sulfasalazine, leflunomide, and hydroxychloroquine. The bDMARDs included were tumor necrosis factor inhibitors (TNFi) and non-TNFi biologics such as tocilizumab, rituximab, abatacept, and biosimilars; tsDMARDs included tofacitinib and baricitinib. Patients with lung cancer before the diagnosis of RA were excluded. Incident lung cancer was determined by ³2 outpatient ICD9/10 codes, or ³1 ICD9/ICD10/ICDO codes if from inpatient or the oncology raw domain. Drug exposure categories (csDMARD versus b/tsDMARD) were treated as a time-varying variable. Multivariable Cox models were fit to estimate the hazard ratio for developing lung cancer during and following the use of a b/tsDMARD relative to b/tsDMARD-naïve persons adjusting for time-varying age, rheumatic disease comorbidity index (RDCI) and RA interstitial lung disease (RA-ILD, identified using validated administrative algorithms(1)); and baseline sex, race, ethnicity, smoking status, body mass index (BMI), cohort entry (2002-2007, 2008-2013, 2014-2019), rheumatoid factor or anti-CCP positivity, use of opioids, NSAIDs, and steroids. Missing data were imputed using multiple imputation by chained equations.
Results: 26,625 veterans met eligibility criteria, of whom 89% were male and 71% were Non-Hispanic White (Table 1). Over a mean (±standard deviation) follow up of 7.0 (±4.5) years, 852 incident lung cancers were detected. The age-standardized incidence among those only on csDMARDs was 4.56 per 1000-person years, compared to 5.33 for those exposed to b/tsDMARDs. In multivariable adjusted models, the hazard ratio for lung cancer in persons who had been treated with a b/tsDMARD was 1.03 (95% confidence interval (CI) 0.87-1.21) relative to those only on csDMARDs (Figure 1).
Conclusion: Among Veterans with RA, we observed no evidence of an altered risk of lung cancer associated with different forms of RA treatment. However, incomplete information on cigarette smoking history, along with the relatively short period of follow-up, limit any conclusions that might be drawn.
Reference:
1. England BR, et al. Performance of Administrative Algorithms to Identify Interstitial Lung Disease in Rheumatoid Arthritis. AC&R 2020
To cite this abstract in AMA style:
Singh N, Peterson A, Baraff A, Wysham K, England B, Baker J, Smith N, Mikuls T, Weiss N. Risk of Lung Cancer in Veterans with Rheumatoid Arthritis in Relation to Use of Conventional versus Biologic or Targeted Synthetic DMARDs [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-lung-cancer-in-veterans-with-rheumatoid-arthritis-in-relation-to-use-of-conventional-versus-biologic-or-targeted-synthetic-dmards/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-lung-cancer-in-veterans-with-rheumatoid-arthritis-in-relation-to-use-of-conventional-versus-biologic-or-targeted-synthetic-dmards/