Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Sodium-glucose cotransporter-2 inhibitors (SGLT2i) are a revolutionary second-line treatment for type 2 diabetes associated with lower risk of cardiovascular and all-cause mortality, heart failure, and chronic kidney disease progression. Many trials have also found SGLT2i lower serum urate levels. However, data on gout risk are limited and the few available prior studies were not specific to those using metformin, the primary target populations of recent landmark trials like GRADE [GRADE Study Research Group, NEJM 2022;387:1075-88].
Our objective was to emulate recent clinical trials and compare incident gout risk among metformin-treated patients with type 2 diabetes initiating SGLT2i versus other second-line type 2 diabetes treatments (dipeptidyl peptidase 4 inhibitors [DPP-4i], glucagon-like peptide-1 receptor agonists [GLP1-RA], or sulfonylureas).
Methods: We performed a new user, active comparator, population-based cohort study using administrative health data for nearly all residents of British Columbia, Canada from Jan 2014 to June 2022, including all dispensed prescriptions, regardless of funder. A cohort of adults with type 2 diabetes using metformin (first-line therapy) was identified from ICD codes and dispensing data. Primary outcome was incident gout, defined as inpatient or outpatient diagnosis of gout plus dispensing of a gout medication (colchicine, corticosteroids, or NSAIDs) within 7 days, and no prior recorded gout diagnosis. We also stratified by sex, age, and baseline diuretic use.
Cox proportional hazards models were used with propensity score overlap weighting, stratified by calendar year of initiation. We also assessed for risk of genital infection (for which we expected SGLT2i would have a positive association), and for the risk of any osteoarthritis encounter, a negative control outcome for which we expected a null association.
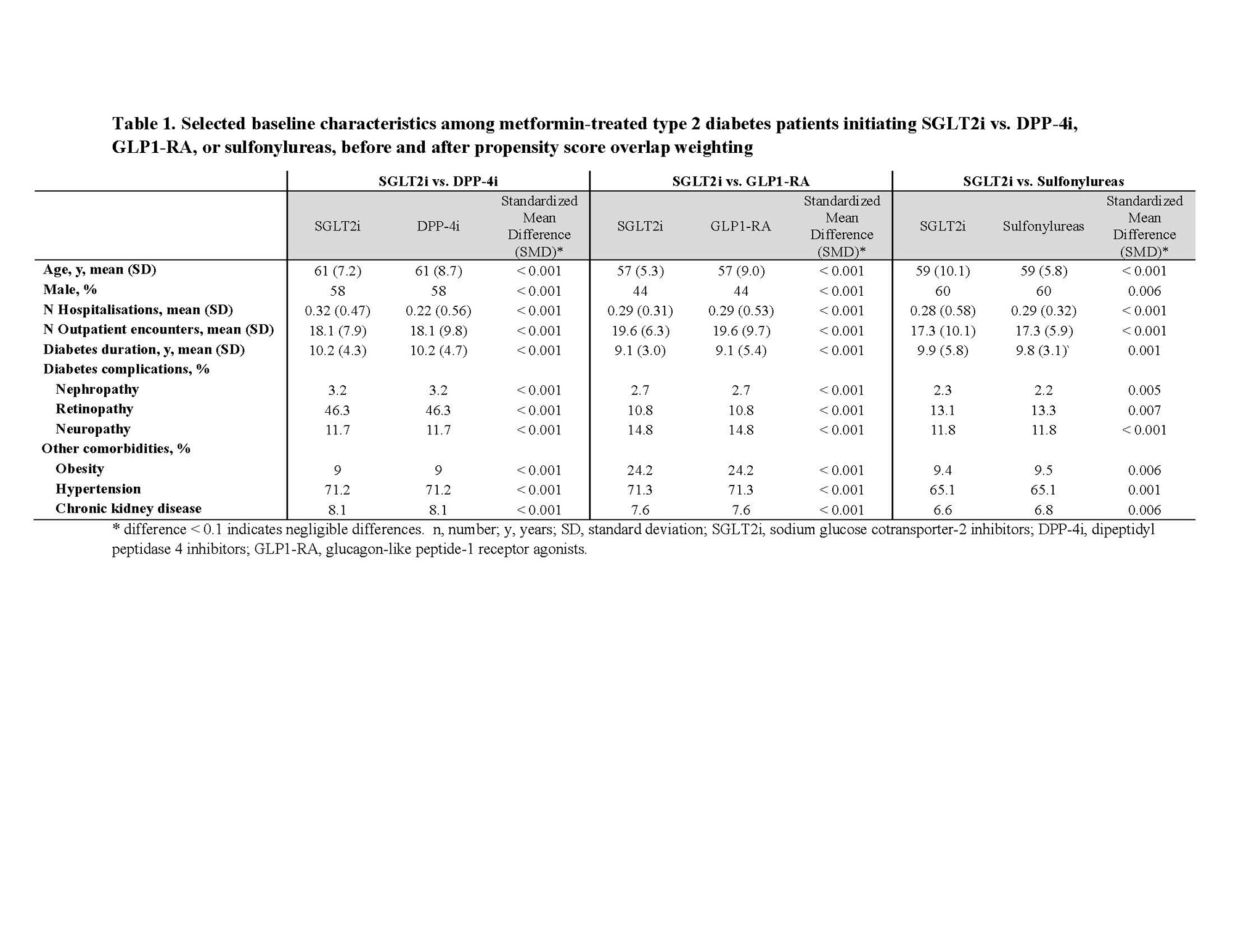
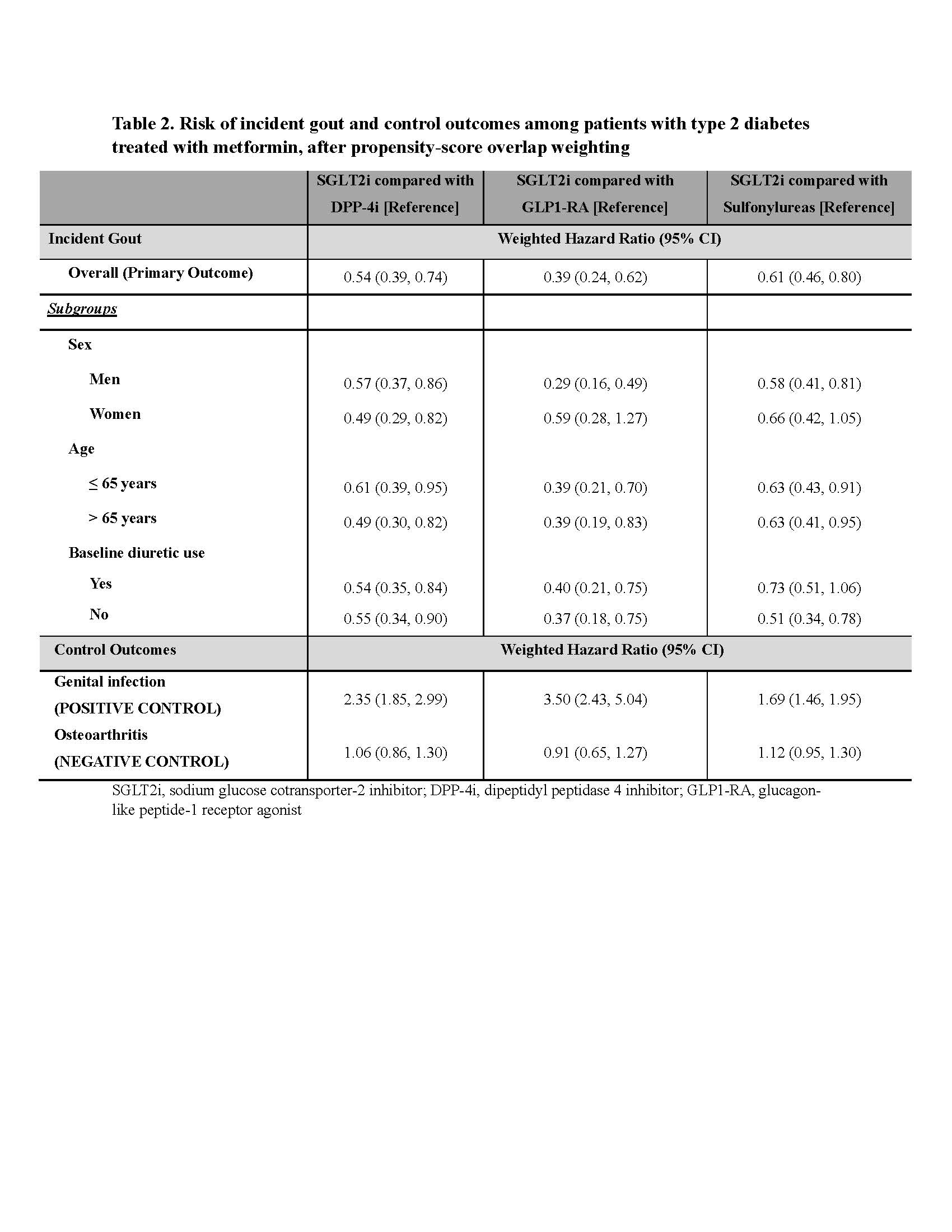
Results: We included 27,791 type 2 diabetes patients in the SGLT2i vs. DPP-4i cohort (58% male, mean age 61), 19,875in the SGLT2i vs. GLP1-RA cohort (44% male, mean age 57), and 71,625in the SGLT2i vs. sulfonylurea cohort (60% male, mean age 59).Baseline characteristics, including diabetes duration and presence of complications, were well balanced between SGLT2i and comparators after overlap weighting (SMD < 0.1) (Table 1).Weighted hazard ratio (wHR) for incident gout associated with SGLT2i initiation was 0.54 (95% CI: 0.39, 0.74) vs. DPP-4i initiation, 0.39 (0.24, 0.62) for SGLT2i vs. GLP1-RA, and 0.61 (0.46, 0.80) for SGLT2i vs. sulfonylureas (Table 2).Results were consistent regardless of sex or age or baseline diuretic use.
For control outcomes, SGLT2i initiators had higher risk of genital infection than initiators of each comparator, as expected, while there was no difference in risk of osteoarthritis (Table 2).
Conclusion: SGLT2i initiation among metformin-treated patients with type 2 diabetes was associated with substantially lower risk of incident gout (i.e., primary prevention), compared with any other second-line option. Along with its known urate-lowering effects, as well as cardiovascular and survival benefits, SGLT2i could substantially reduce risk of incident gout for patients needing a second-line agent after metformin.
* difference < 0.1 indicates negligible differences. n, number; y, years; SD, standard deviation; SGLT2i, sodium glucose cotransporter_2 inhibitors; DPP_4i, dipeptidyl peptidase 4 inhibitors; GLP1-RA, glucagon-like peptide_1 receptor agonists.
SGLT2i, sodium glucose cotransporter_2 inhibitor; DPP_4i, dipeptidyl peptidase 4 inhibitor; GLP1-RA, glucagon-like peptide_1 receptor agonist
To cite this abstract in AMA style:
McCormick N, Yokose C, Lu N, Choi H. Risk of Incident Gout Associated with Initiation of Sodium-glucose cotransporter-2 Inhibitors versus Other Second-line Agents Among Metformin Users with Type 2 Diabetes [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-of-incident-gout-associated-with-initiation-of-sodium-glucose-cotransporter-2-inhibitors-versus-other-second-line-agents-among-metformin-users-with-type-2-diabetes/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-incident-gout-associated-with-initiation-of-sodium-glucose-cotransporter-2-inhibitors-versus-other-second-line-agents-among-metformin-users-with-type-2-diabetes/