Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Safety evidence of biological or targeted synthetic DMARDs (b/tsDMARDs) is still insufficient in older elderly ( >=75 years old (y/o)) patients with rheumatoid arthritis (RA) , who are usually excluded from randomized clinical trials. In superaged societies, accumulation of safety data in the real world, especially those of infectious events, is one of the most pressing challenges. We therefore compared risks of hospitalized infections (HIs) across b/tsDMARDs in patients with RA in various age groups.
Methods: A retrospective longitudinal population-based study was conducted using a Japanese claims data provided by Medical Data Vision Co., Ltd. (Tokyo, Japan). We defined individuals as users of b/tsDMARDs if they met all of the following: 1) having at least one ICD10 code (M05 or M06 [excluding M06.1]); 2) having at least one prescription of b/tsDMARDs between October 10, 2014 and February 28, 2019; 3) having data prior to 12 months before the index month (baseline). The index month was defined as the first month of the prescription of b/tsDMARDs during the above term. Patients were excluded from the study population if they had a claim of at least one of the diseases except for RA with indications of b/tsDMARDs during the baseline. Patients were followed from the index month until the last exposure to b/tsDMARDs started in the index month, date of loss of follow-up, or the end of follow-up (February 2019), whichever came first. HIs were defined by ICD10 code with one prescription of predefined drugs for each infection during hospitalizations. Some of HIs were defined by ICD10 code alone. To compare the risk of HIs across b/tsDMARDs, adjusted risk ratios (aRRs) with 95% confidence intervals (95% CI) adjusted for sex, age, year of treatment start, comorbidity, glucocorticoids use, methotrexate use, and treatment duration were calculated using a Poisson regression model. Multivariable analyses were conducted in the patients ≥75 y/o, ≥65 and < 75 y/o, and < 65 y/o separately.
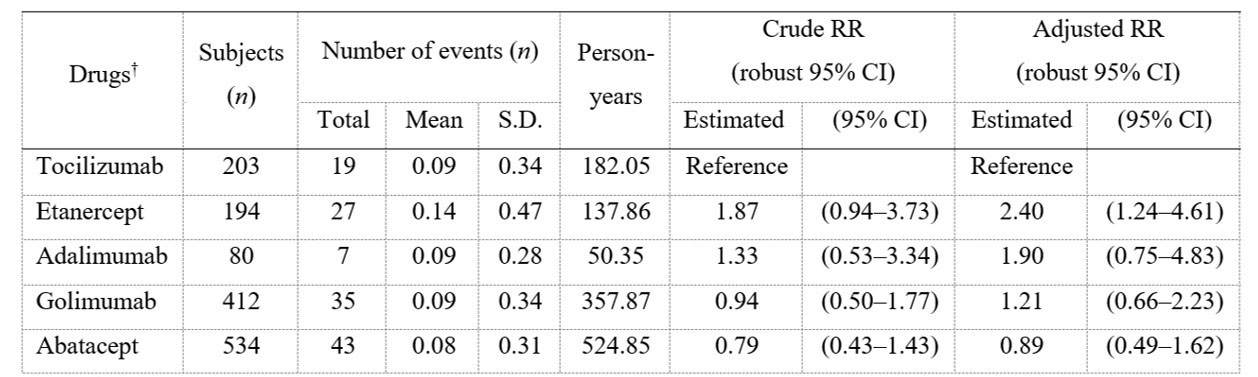
Results: Of 5506 patients enrolled, 2265 (41.2%), 1709 (31.0%), and 1532 (27.8%) were < 65, 65–74, and ≥75 y/o, respectively. Crude incidence rates of HIs (/100PY) were 3.99, 7.27, and 10.77 for < 65, 65–74, and ≥75 y/o, respectively. Among patients ≥75 y/o, aRRs (95% CI) of each bDMARD vs tocilizumab (TCZ) for HIs were as follows: etanercept 2.40 (1.24–4.61); adalimumab 1.90 (0.75–4.83); golimumab 1.21 (0.66–2.23); and abatacept 0.89 (0.49–1.62) (Table 1). For the analysis by drug mechanism of action, aRRs of other bDMARDs groups vs IL-6 inhibitors for HI in patients ≥75 y/o did not show a noticeable increase or decrease (Table 2). In patients < 65 years old, aRR of etanercept vs TCZ for HI was 0.30 (0.11–0.85). aRRs of other b/tsDMARDs vs TCZ for HI in patients < 65 y/o and of all b/tsDMARDs in patients aged 65–74 y/o as well as overall population did not show a noticeable increase or decrease.
Conclusion: In patients with RA ≥75 y/o, the risk of HIs in patients treated with TCZ was not different from patients treated with adalimumab, golimumab and abatacept, and lower than patients treated with etanercept.
† b/tsDMARDs used in <50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
† Each b/tsDMARDs category used in <50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
To cite this abstract in AMA style:
Harigai M, Fujii T, Sakai R, Igarashi A, Shoji A, Yamaguchi H, Iwasaki K, Makishima M, Yoshida A, Okada N, Yamashita K, Kawahito Y. Risk of Hospitalized Infections in Older Elderly Rheumatoid Arthritis Patients Treated with Biological/Targeted Synthetic DMARDs: Evaluation Using Data from a Japanese Claims Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/risk-of-hospitalized-infections-in-older-elderly-rheumatoid-arthritis-patients-treated-with-biological-targeted-synthetic-dmards-evaluation-using-data-from-a-japanese-claims-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-hospitalized-infections-in-older-elderly-rheumatoid-arthritis-patients-treated-with-biological-targeted-synthetic-dmards-evaluation-using-data-from-a-japanese-claims-database/