Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Prophylactic anti-viral therapy is required in patients with hepatitis B virus (HBV) infection receiving biologics because of the high risk of HBV reactivation. However, it is unclear when to start biologics after anti-viral prophylaxis. We investigated the risk of HBV reactivation according to the timing of biologics initiation after anti-HBV prophylaxis in IMID patients with HBV infection.
Methods: We retrospectively evaluated the incidence of HBV reactivation in IMID patients who received biologics between July 2005 and April 2020. The patients were divided into two groups (“within 1-week ” vs. “after 1-week”) according to the timing of biologics initiation after anti-HBV prophylaxis. The cumulative probabilities and factors associated with HBV reactivation were evaluated.
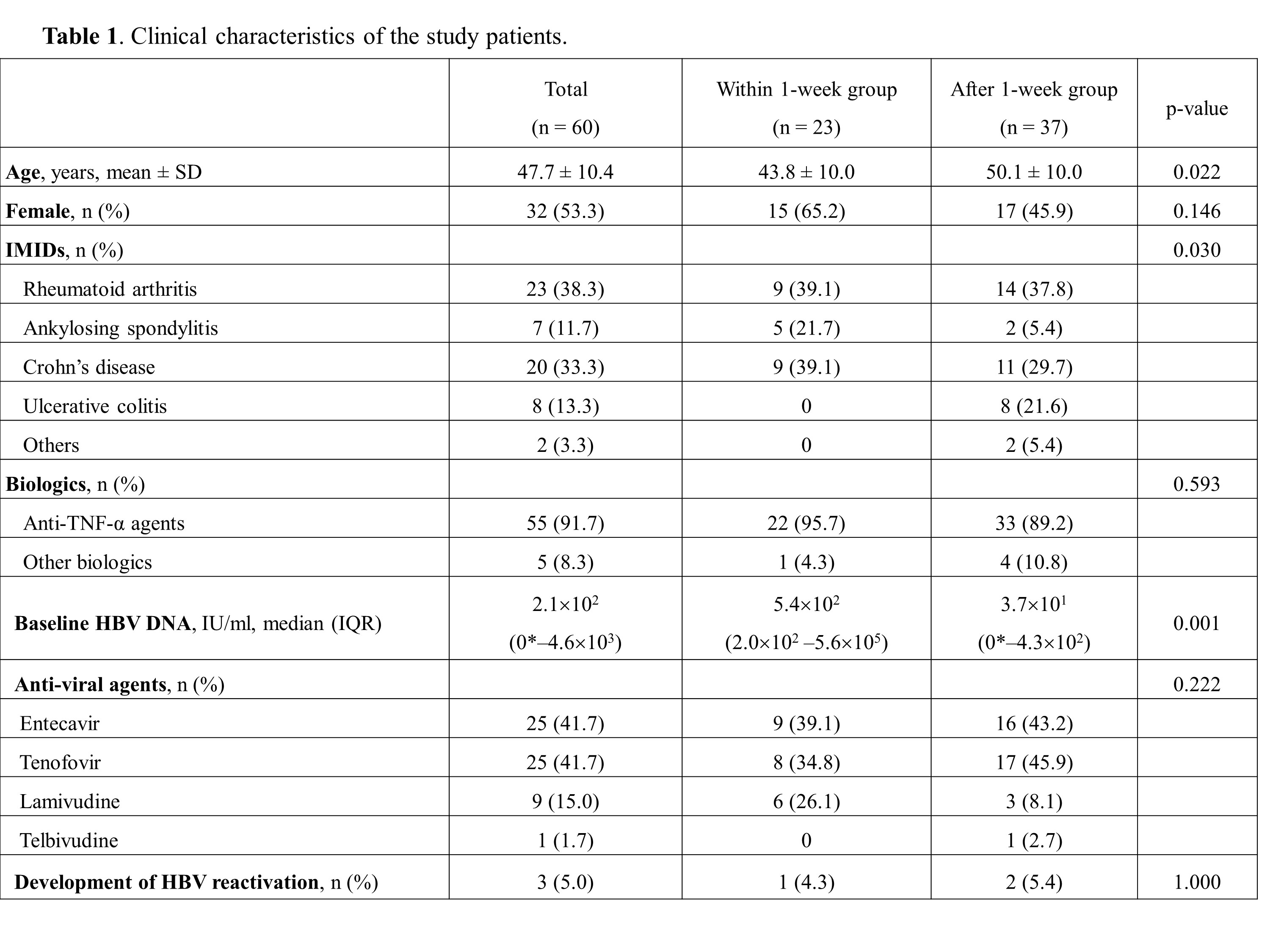
Results: A total of 60 hepatitis B surface antigen-positive patients with IMID received biologics (within 1-week group, n = 23 [38%]; after 1-week group, n = 37 [62%]). During a median follow-up of 34 months (interquartile range, 20–74), three (5%) patients developed HBV reactivation. In the univariate analysis, the timing of biologics after HBV prophylaxis was not significantly associated with the risk of HBV reactivation (hazard ratio, 0.657; 95% confidence interval, 0.059–7.327; p = 0.733). The cumulative probabilities of HBV reactivation did not significantly differ according to the timing of biologics as well (p = 0.731).
Conclusion: The risk of HBV reactivation was not significantly associated with the timing of biologics administration after anti-HBV prophylaxis. Thus, biologics may be initiated early in patients with IMID under prophylactic treatment for HBV.
To cite this abstract in AMA style:
Ahn S, Choi J, Ye B, Yang S, Oh J, Kim Y, Lee C, Yoo B, Park S, Hong S. Risk of Hepatitis B Virus Reactivation in Patients with Immune-Mediated Inflammatory Diseases Receiving Biologics: Focus on the Timing of Biologics After Prophylactic Anti-viral Agents [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/risk-of-hepatitis-b-virus-reactivation-in-patients-with-immune-mediated-inflammatory-diseases-receiving-biologics-focus-on-the-timing-of-biologics-after-prophylactic-anti-viral-agents/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-hepatitis-b-virus-reactivation-in-patients-with-immune-mediated-inflammatory-diseases-receiving-biologics-focus-on-the-timing-of-biologics-after-prophylactic-anti-viral-agents/