Session Information
Date: Sunday, November 7, 2021
Title: Plenary II (0956–0961)
Session Type: Plenary Session
Session Time: 11:00AM-11:15AM
Background/Purpose: To identify independent risk factors for major adverse cardiovascular (CV) events (MACE) in ORAL Surveillance (NCT02092467), a long-term, randomized, open-label, non-inferiority, Phase 3b/4 safety study of tofacitinib vs TNFi in high-risk (aged ≥ 50 yrs with ≥ 1 additional CV risk factor) patients (pts) with RA and an inadequate MTX response.
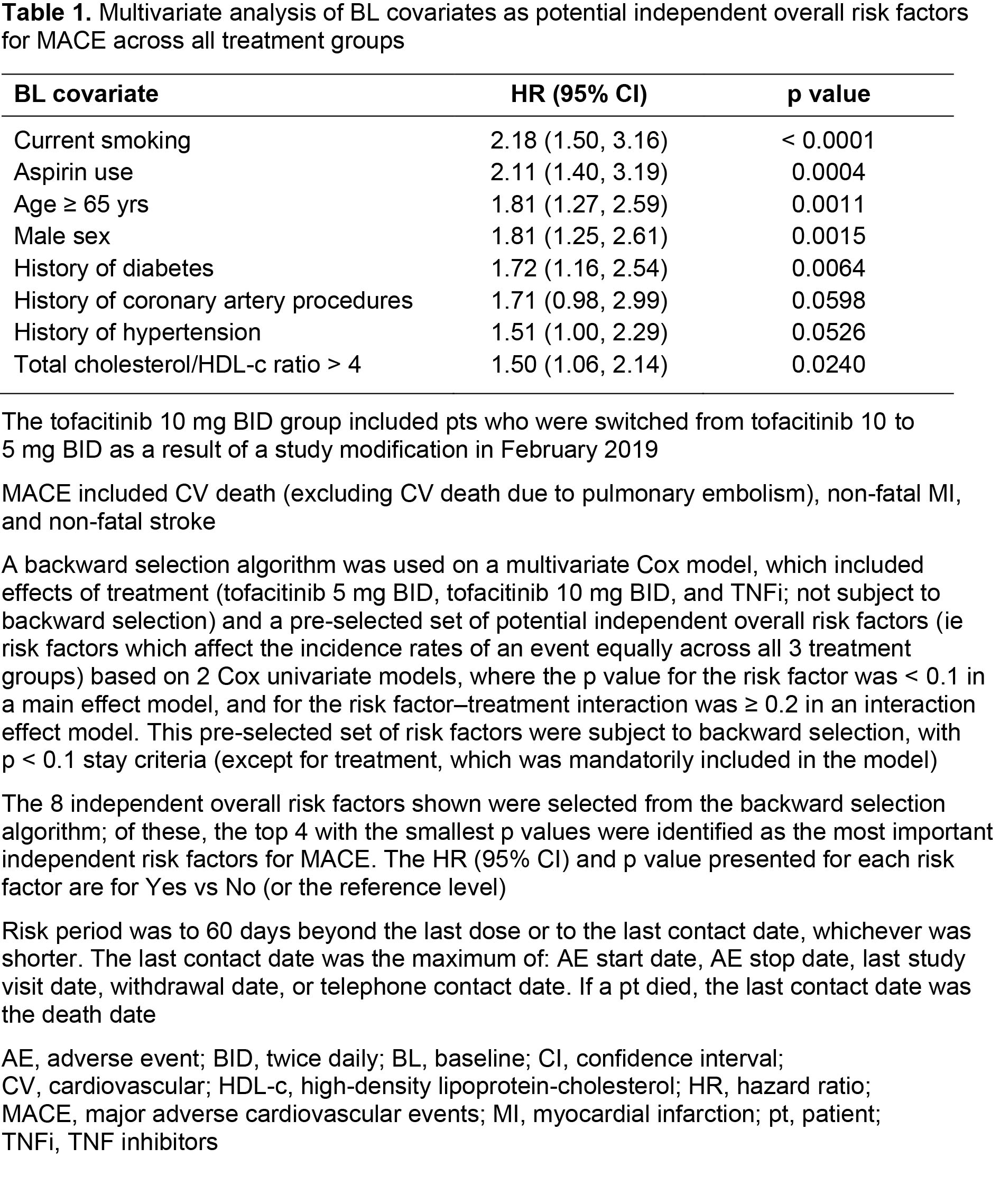
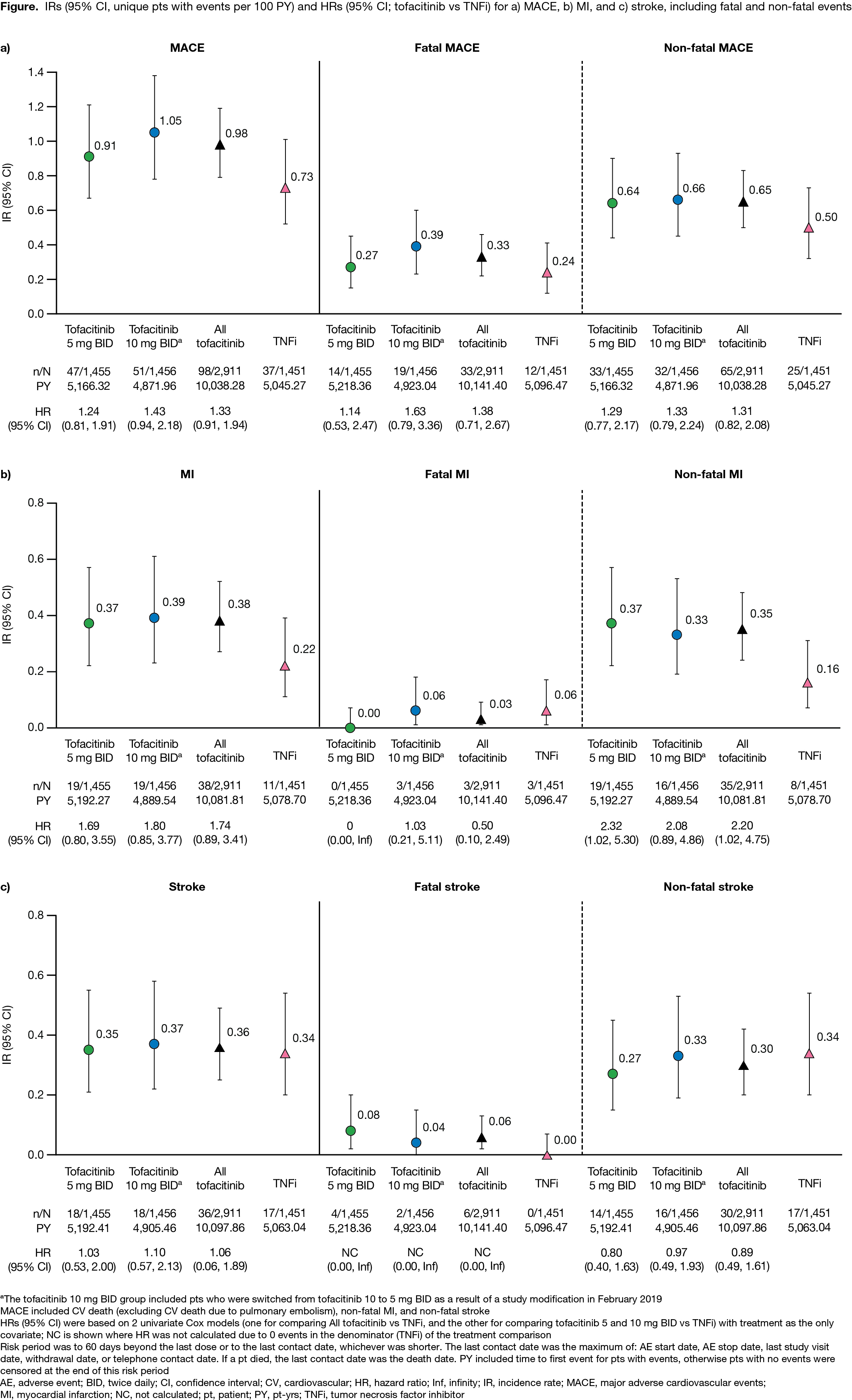
Methods: Pts on stable doses of MTX were randomized 1:1:1 to receive tofacitinib 5 or 10 mg BID or a TNFi (adalimumab 40 mg every 2 weeks or etanercept 50 mg every week). Incidence rates (IRs; unique pts with events/100 pt-yrs) and hazard ratios (HRs; tofacitinib vs TNFi) were evaluated for the co-primary endpoint of adjudicated MACE, as well as myocardial infarction (MI) and stroke; MACE was defined as CV death (excluding CV death due to pulmonary embolism), non-fatal MI, and non-fatal stroke. Across outcomes, IRs and HRs are also presented for fatal and non-fatal events. A post hoc analysis identified independent overall risk factors for MACE across treatment groups by Cox regression models. Baseline (BL) categorical covariates were initially screened in 2 univariate Cox analyses (main risk factor p < 0.1 and risk factor–treatment interaction p ≥ 0.2) and were then selected in the final multivariate Cox model using backward selection, with p < 0.1 stay criteria. IRs and HRs for MACE were then stratified by the 4 most important risk factors identified (per largest HRs and smallest p values in the multivariate Cox model).
Results: 4,362 pts were included (tofacitinib 5 mg BID, n=1,455; tofacitinib 10 mg BID, n=1,456; TNFi, n=1,451). Median (range) age was 60.0 (50.0–88.0) yrs. MACE was reported by 47 pts, 51 pts, and 37 pts receiving tofacitinib 5 mg BID, tofacitinib 10 mg BID, and TNFi, respectively (Figure). IRs for MACE, MI, fatal and non-fatal MACE, and non‑fatal MI were numerically higher with both tofacitinib doses vs TNFi (95% CIs overlapped; Figure a, b). Generally, IRs for fatal MI, and stroke (including fatal/non-fatal events), were similar across treatment groups (Figure a–c). HRs for MACE, MI, and stroke were generally > 1 for tofacitinib vs TNFi (Figure a–c); HR 95% CIs for non-fatal MI excluded 1 for tofacitinib 5 mg BID and all tofacitinib vs TNFi. BL covariates identified as the most important independent overall risk factors for MACE were current smoking, aspirin use, age ≥ 65 yrs, and male sex (Table 1). In pts with 1 of these risk factors present, IRs for MACE were numerically higher with both tofacitinib doses vs TNFi (95% CIs overlapped; Table 2). Across treatment groups, in pts without these risk factors present, IRs for MACE were similar (Table 2). Generally, HRs for MACE were > 1 for tofacitinib vs TNFi, in pts with or without risk factors present (Table 2).
Conclusion: The incidence of MACE was numerically higher with tofacitinib vs TNFi treatment. Across treatment groups, the BL covariates, current smoking, aspirin use, age ≥ 65 yrs, and male sex were identified as independent overall risk factors in pts experiencing MACE; tofacitinib vs TNFi treatment in pts with any of these risk factors present was associated with numerically higher incidence of MACE.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by K Woollcott, CMC Connect, funded by Pfizer Inc.
To cite this abstract in AMA style:
Charles-Schoeman C, Buch M, Dougados M, Bhatt D, Giles J, Vranic I, Wu J, Wang C, Menon S, Rivas J, Yndestad A, Connell C, Szekanecz Z. Risk Factors for Major Adverse Cardiovascular Events in Patients Aged ≥ 50 Years with RA and ≥ 1 Additional Cardiovascular Risk Factor: Results from a Phase 3b/4 Randomized Safety Study of Tofacitinib vs TNF Inhibitors [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-major-adverse-cardiovascular-events-in-patients-aged-%e2%89%a5-50-years-with-ra-and-%e2%89%a5-1-additional-cardiovascular-risk-factor-results-from-a-phase-3b-4-randomized-safety-stud/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-major-adverse-cardiovascular-events-in-patients-aged-%e2%89%a5-50-years-with-ra-and-%e2%89%a5-1-additional-cardiovascular-risk-factor-results-from-a-phase-3b-4-randomized-safety-stud/