Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Adalimumab is a monoclonal antibody against TNF-α that is commonly used to treat JIA, hidradenitis suppurativa (HS), chronic uveitis, sarcoidosis, and chronic nonbacterial osteomyelitis (CNO). Anti-drug antibodies (ADAs) have been frequently reported in patients receiving adalimumab, leading to decreased therapeutic efficacy of the drug. Identifying clinical factors associated with ADA formation is essential to optimize long-term outcomes. This study aims to evaluate differences in treatment duration, flare frequency, and methotrexate use between patients with and without ADAs to adalimumab.
Methods: With IRB approval, a retrospective cohort study was done to evaluate ADA formation in pediatric patients receiving adalimumab for JIA, CNO, uveitis, sarcoidosis, or HS at a tertiary care center. A total of 119 patients were stratified into two groups based on presence or absence of ADAs. Data collected included duration of adalimumab therapy, dose frequency, flare frequency, methotrexate co-therapy, and treatment interruptions. Differences between groups were determined by chi-square test for categorical variables and Kruskal-Wallis test for continuous variables. A p-value of < 0.05 was considered statistically significant.
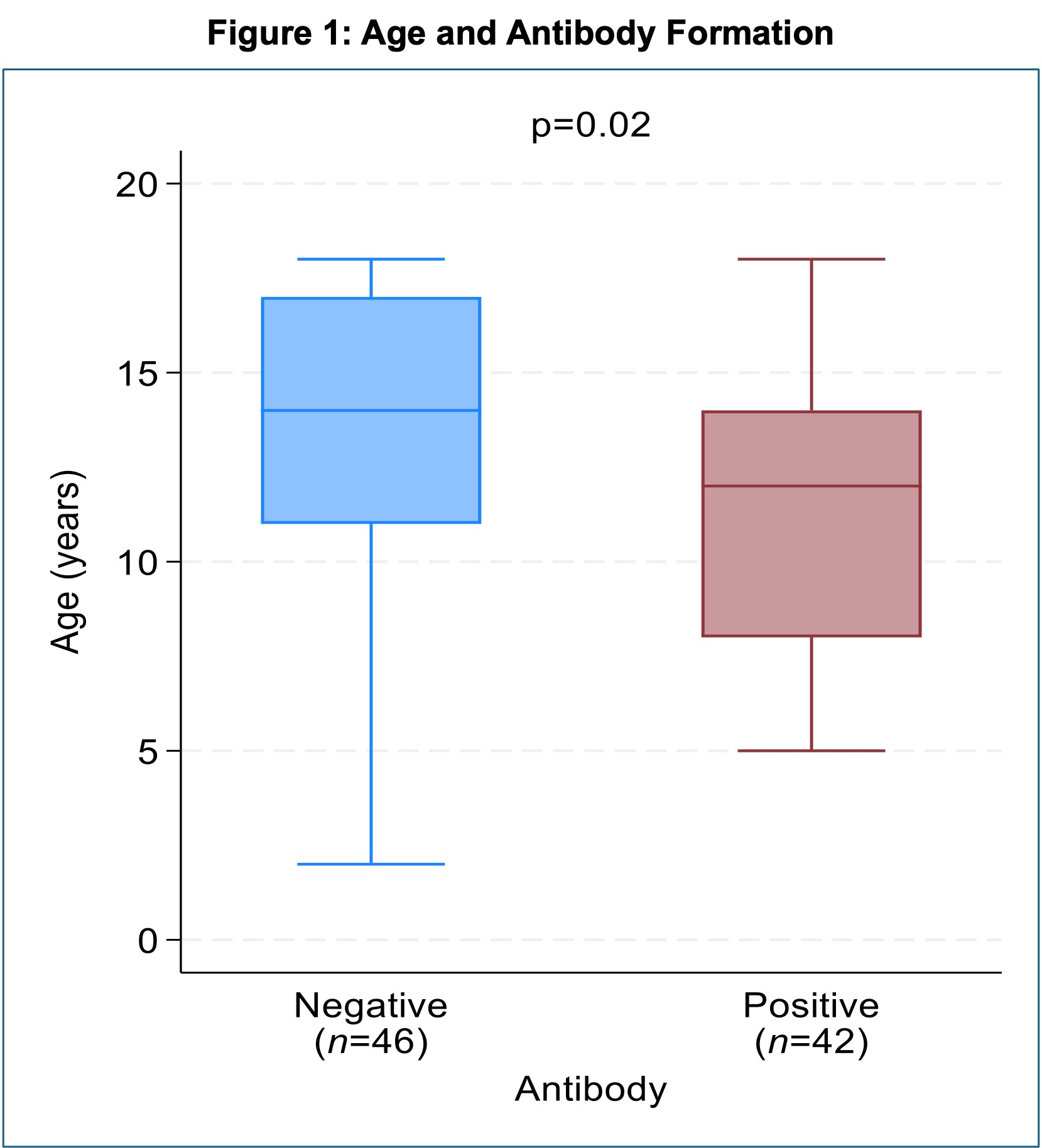
Results: Among 119 patients, 88 received adalimumab every 14 days, 21 every 7 days, and 10 at other frequencies. Patients on treatment every 7 days had less antibody formation than those on every 14 days (p = 0.04). Further analysis was done on the 88 patients on every 14-day regimen. Of those, 42 (37.17%) developed ADAs (Table 1). Patients with ADAs were of younger age than those without ADAs (12 years vs. 14 years, p = 0.02) (Figure 1). Flare rates were higher in patients with ADAs (69.86% vs. 97.6%; p = 0.001). The median duration of adalimumab use was not statistically different, though still greater in those with ADAs compared to those without (24 months [IQR 13–47] vs. 13 months [IQR 7–33]; p = 0.02) (Figure 2). Methotrexate was used more frequently in the ADA-negative group (43.5% vs. 21.4%, p = 0.049). Dosing of methotrexate in mg/m2 was not different between the groups. Treatment interruptions of >1 month occurred in 33.3% of ADA-positive patients vs. 17.4% of ADA-negative patients (p = 0.14), and use of methotrexate during these interruptions did not differ.
Conclusion: ADA development was more common in patients not receiving methotrexate and was associated with increased disease flares. Although length of therapy and treatment interruption did not differ significantly, the data suggests a possible role in immunogenicity risk. Patients with frequent adalimumab dosing regimens were less likely to develop ADAs, though use of methotrexate was not analyzed in this increased frequency group and needs exploration. Younger age was associated with increased risk of antibody development. Results suggest that early use of ADA monitoring, particularly in younger patients and those on less frequent adalimumab regimens, may be helpful. The addition of methotrexate therapy or increasing the dosing frequency in patients who cannot tolerate DMARDs may help reduce immunogenicity and improve clinical outcomes in pediatric rheumatology.
 Patient characteristics by adalimumab antibody positivity, including age, sex, race, ethnicity, diagnosis, months on adalimumab, concurrent methotrexate use, dosing of methotrexate, flare rate, interruptions in adalimumab therapy, and use of methotrexate during adalimumab therapy interruptions.
Patient characteristics by adalimumab antibody positivity, including age, sex, race, ethnicity, diagnosis, months on adalimumab, concurrent methotrexate use, dosing of methotrexate, flare rate, interruptions in adalimumab therapy, and use of methotrexate during adalimumab therapy interruptions.
.jpg) Box and whisker plot of age (in years) at the time of antibody testing compared between positive and negative adalimumab antibody groups.
Box and whisker plot of age (in years) at the time of antibody testing compared between positive and negative adalimumab antibody groups.
.jpg) Box and whisker plot of the number of months on adalimumab compared between positive and negative adalimumab antibody groups.
Box and whisker plot of the number of months on adalimumab compared between positive and negative adalimumab antibody groups.
To cite this abstract in AMA style:
Gist D, Ramirez A, Lai J, Nguyen D, Guo K. Risk Factors for Anti-Adalimumab Antibody Development in Pediatric Patients Using Adalimumab for Rheumatic Disease and Associated Conditions [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/risk-factors-for-anti-adalimumab-antibody-development-in-pediatric-patients-using-adalimumab-for-rheumatic-disease-and-associated-conditions/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-for-anti-adalimumab-antibody-development-in-pediatric-patients-using-adalimumab-for-rheumatic-disease-and-associated-conditions/
