Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Immune checkpoint inhibitors (ICI) stimulate the immune system to treat cancer but may cause flares of pre-existing immune-mediated inflammatory diseases. Risk factors for ICI-induced RA flares are unknown. Some studies suggest that flare of pre-existing autoimmune disease after ICI may portend a mortality benefit, perhaps as a marker of immune activity against cancer, but this has not been investigated for ICI-induced RA flares. Evaluating a post-baseline outcome such as RA flare could be prone to immortal time bias where patients must survive long enough to flare, falsely showing an association with mortality. Therefore, we studied risk factors and outcomes of ICI-induced RA flares, considering competing risk of death and immortal time bias in the analysis.
Methods: We performed a retrospective cohort study of patients with pre-existing RA initiating ICI for cancer treatment at a large healthcare system (2011-2022). We performed medical record review on patients with a RA diagnosis code prior to ICI initiation to confirm patients met 2010 ACR/EULAR RA criteria. We collected data on demographics, cancer, RA, RA disease activity/flares after ICI initiation, ICI disruption, and mortality. We analyzed baseline risk factors for RA flare after ICI initiation taking into account competing risk of death using the Fine and Gray method. We performed a landmark analysis to limit the potential for immortal time bias, where baseline was 3 months after ICI initiation. Among those who survived at least 3 months, we examined whether RA flare within 3 months after ICI initiation was associated with mortality using Cox regression.
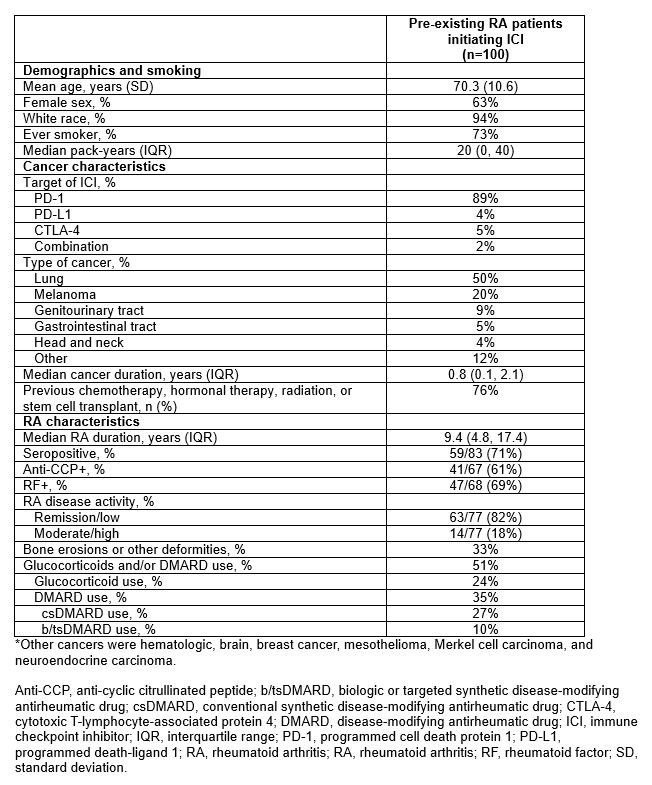
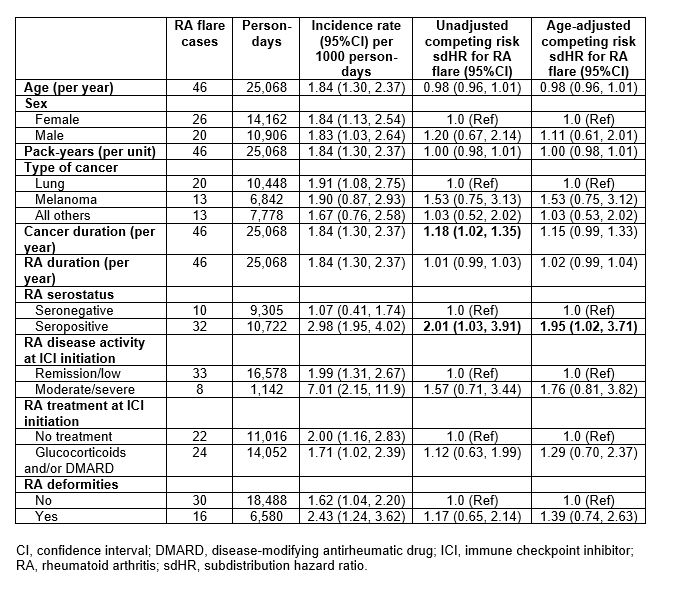
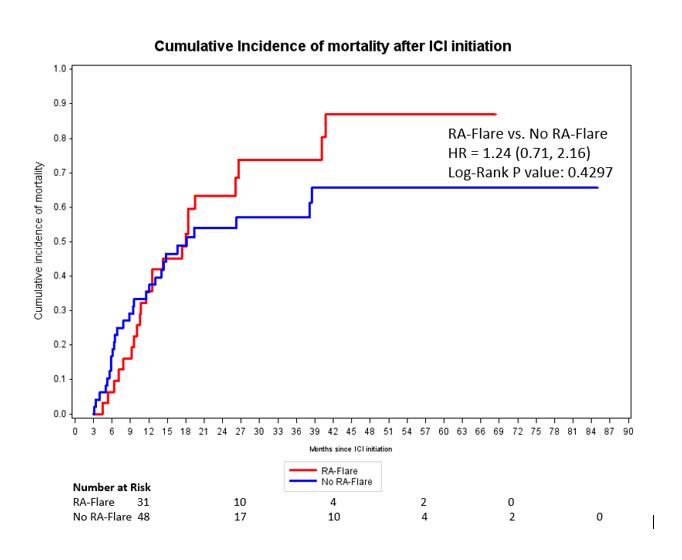
Results: Among 11,901 patients who initiated ICI for cancer treatment, we analyzed 100 pre-existing RA patients (mean age 70.3 years, 63% female, 89% on PD-1 monotherapy, 50% lung cancer). At ICI initiation, 71% were seropositive, 82% had remission/low RA disease activity, 24% were on glucocorticoids, 35% were on conventional DMARDs, and 10% were on b/tsDMARDs (Table 1). RA flares occurred in 46% (incidence rate 1.84 per 1000 person-days, 95%CI 1.30, 2.37); 31/100 flared within 3 months of baseline. 72% died during follow-up, 21/100 within 3 months of baseline. Seropositive RA had an adjusted sdHR of 1.95 (95%CI 1.02, 3.71) for RA flare compared to seronegative, accounting for competing risk of death (Table 2). Otherwise, there were no baseline factors associated with RA flare, including cancer type, baseline disease activity, RA duration, and deformities. 9/46 (20%) patients had their ICI discontinued/paused due to RA flares. In the landmark analysis among 79 patients who survived at least 3 months, RA flare in the first 3 months was not associated with lower mortality (adjusted HR 1.24, 95%CI 0.71, 2.16) compared to no RA flare (Figure).
Conclusion: RA flares and mortality were each common among pre-existing RA patients initiating ICI for cancer. Seropositivity was the only baseline factor associated with RA flare after accounting for the competing risk of death. RA flare after ICI may not have prognostic implications for mortality. Previous findings suggesting mortality benefit from flares of other autoimmune diseases after ICI may have been due to immortal time bias.
To cite this abstract in AMA style:
McCarter K, Arabelovic S, Wolfgang T, Wang X, Yoshida K, Qian G, Kowalski E, Vanni K, LeBoeuf N, Buchbinder E, Gedmintas L, MacFarlane L, Rao D, Shadick N, Gravallese E, Sparks J. Risk Factors and Mortality of Immune Checkpoint Inhibitor-Induced Flares of Pre-Existing Rheumatoid Arthritis: An Analysis Accounting for Competing Risk of Death and Immortal Time Bias [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/risk-factors-and-mortality-of-immune-checkpoint-inhibitor-induced-flares-of-pre-existing-rheumatoid-arthritis-an-analysis-accounting-for-competing-risk-of-death-and-immortal-time-bias/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-factors-and-mortality-of-immune-checkpoint-inhibitor-induced-flares-of-pre-existing-rheumatoid-arthritis-an-analysis-accounting-for-competing-risk-of-death-and-immortal-time-bias/