Session Information
Date: Monday, November 8, 2021
Session Type: Abstract Session
Session Time: 4:00PM-4:15PM
Background/Purpose: Recurrent pericarditis (RP) is an autoinflammatory disease with no FDA-approved therapies. RHAPSODY, a global Phase 3 study, evaluated rilonacept, a once-weekly IL-1α/IL-1β trap, in RP. RHAPSODY data helped support FDA approval of the first therapy for RP.
Methods: RHAPSODY was a randomized withdrawal (RW) trial: 86 patients (pts) with acute symptomatic RP, failing standard therapy (NSAIDs, colchicine, and/or corticosteroids [CS]), enrolled in a 12-week single-blind run-in (RI) period: rilonacept (320 mg SC load, 160 mg SC weekly) was administered while background pericarditis medications were discontinued. Clinical responders on rilonacept monotherapy were then randomized 1:1 to placebo or continued rilonacept in an event-driven double-blind RW period. Primary efficacy endpoint: time-to-first adjudicated pericarditis recurrence. Pts experiencing an eligible RP event during RW could receive open-label (OL) bailout rilonacept and remain on study.
Results: Etiology: idiopathic (85%), post-cardiac injury (15%).
Baseline characteristics (mean): disease duration was 2.4 years, with 4.7 lifetime episodes and 4.4 episodes/year.
Qualifying episode: pericarditis medications ─ NSAIDs (67%), colchicine (80%), and CS (49%). Mean pericarditis pain (11-point NRS scale) was 6.2, and mean C-reactive protein (CRP) was 6.18 mg/dL.
RI: 92% of pts completed RI; medians: time to NRS score ≤2 was 5 days; time to CRP normalization (≤0.5 mg/dL) was 7 days; time to Treatment Response was 5 days; time to monotherapy rilonacept was 7.9 weeks.
RW: 25 adjudicated events accrued in 61 randomized pts (Figure). Median time to event: placebo 8.6 wks, rilonacept Not-Estimable due to few events; HR 0.04 (96% risk reduction) (p < 0.0001). All 3 major secondary efficacy endpoints (Wk 16) were significant: maintained Clinical Response (rilonacept 81% vs. placebo 20% [p=0.0002]); absent/minimal pericarditis symptoms on 7-point Patient Global Impression of Pericarditis Severity scale (rilonacept 81% vs. placebo 25% [p=0.0006]); % of days with none/minimal pericarditis pain (rilonacept 98% vs. placebo 46% [p < 0.0001]).
No subsequent RP events were documented in pts on OL bailout rilonacept. 74 of 75 eligible pts continue OL rilonacept in a long-term extension up to 24 months.
Rilonacept was well-tolerated; 4 discontinuations in RI for AEs; SAEs: rilonacept (n=4), placebo (n=1), none drug-related; 1 withdrawn consent in RW.
Conclusion: Rilonacept (IL-1α/IL-1β trap) provided rapid and sustained reductions in pain and CRP as soon as after the first dose. Rilonacept monotherapy reduced RP event risk by 96% vs placebo. Rilonacept may provide a targeted therapeutic option for patients with RP.
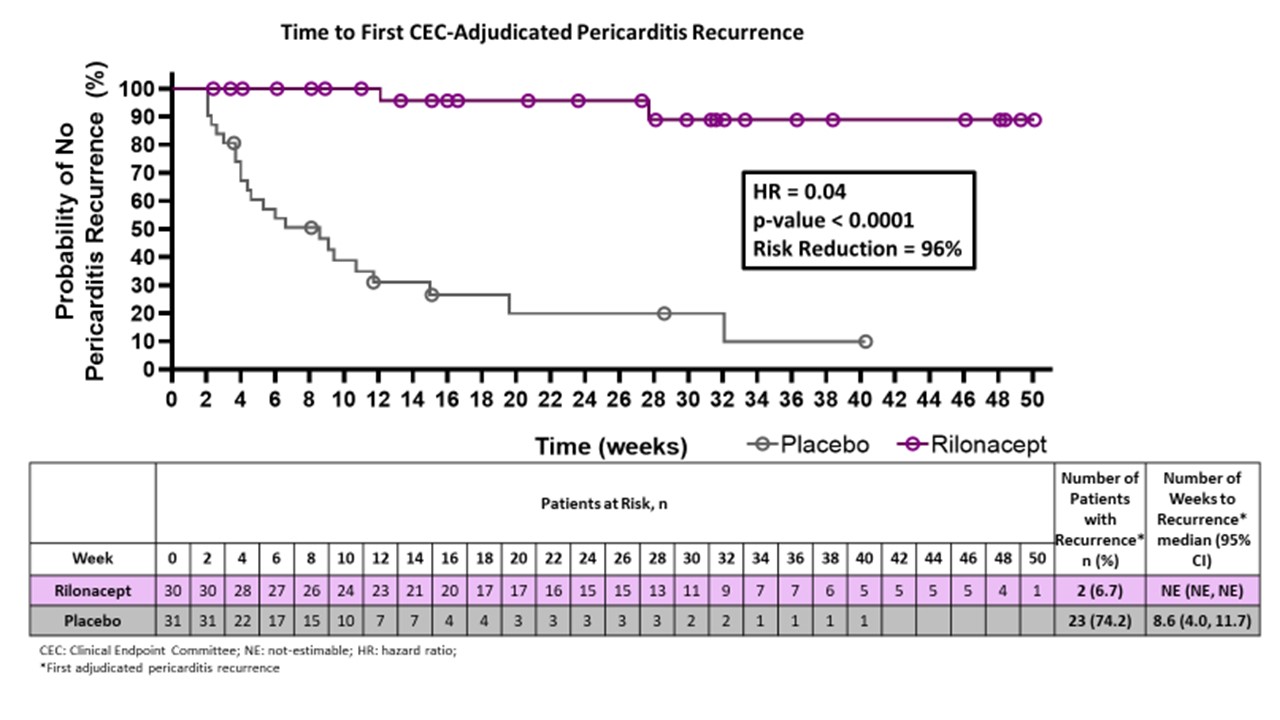 Figure. Time to First CEC-Adjudicated Pericarditis Recurrence
Figure. Time to First CEC-Adjudicated Pericarditis Recurrence
To cite this abstract in AMA style:
Klein A, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, Insalaco A, LeWinter M, Lewis B, Lin D, Luis S, Nicholls S, Pano A, Wheeler A, Zou L, Paolini J. RHAPSODY: Rilonacept, an IL-1α and IL-1β Trap, Resolves Pericarditis Episodes and Reduces Risk of Recurrence in a Phase 3 Trial of Patients with Recurrent Pericarditis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/rhapsody-rilonacept-an-il-1%ce%b1-and-il-1%ce%b2-trap-resolves-pericarditis-episodes-and-reduces-risk-of-recurrence-in-a-phase-3-trial-of-patients-with-recurrent-pericarditis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rhapsody-rilonacept-an-il-1%ce%b1-and-il-1%ce%b2-trap-resolves-pericarditis-episodes-and-reduces-risk-of-recurrence-in-a-phase-3-trial-of-patients-with-recurrent-pericarditis/
