Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Neutropenia has been reported as one of the most frequent adverse events of tocilizumab. We conducted a retrospective cohort study to determine the risks and clinical significance of tocilizumab-induced neutropenia, in real-world settings, for patients with rheumatoid arthritis (RA).
Methods: We collected the medical records of patients with RA who were treated with tocilizumab, between January 2013 and December 2021, at a tertiary referral hospital in Seoul, South Korea. Data regarding complete blood cell counts were collected. Neutropenia was graded following the Common Terminology Criteria for Adverse Events guidelines as follows: grade 0, normal; grade 1, < lower limit of normal (LLN) to 1.5 × 109/L; grade 2, < 1.5 to 1.0 × 109/L; grade 3, < 1.0 to 0.5 × 109/L; and grade 4, < 0.5 × 109/L. Infectious complications were confirmed by clinical diagnosis and treated with antibiotics.
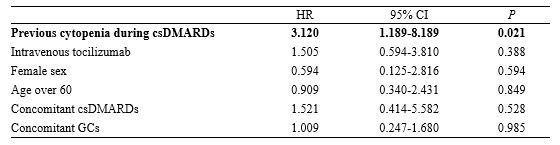
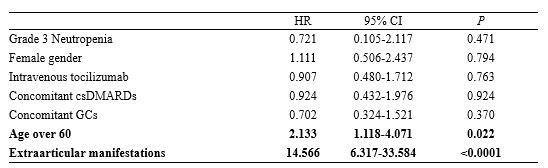
Results: A total of 277 patients with RA, who received tocilizumab treatment, were included in our study. Of the total patients, tocilizumab was administered as subcutaneous and intravenous injections in 152 (54.8%) and 125 (45.1%) patients, respectively, during 782 patient-years (PY). During the follow-up period, 22 (7%) patients experienced grade 3/4 neutropenia. None of the patients discontinued tocilizumab due to neutropenia, while the dosage of conventional synthetic DMARD (csDMARD) was either reduced or discontinued for 8 patients. There was no significant difference in the time taken for recovery from neutropenia, between the csDMARD discontinuation group (median, 1.6 mo [IQR: 1.0–2.0]) and the csDMARD continuation group (median, 2.6 mo [IQR: 1.0–5.3]) (P=0.58). A previous history of neutropenia during treatment with csDMARD could contribute to a higher risk for onset of grade 3/4 neutropenia (HR: 3.120; 95% CI: 1.189-8.189; P=0.021). In the enrolled population, infections occurred during tocilizumab treatment primarily included pulmonary infections at 10.31/100 PY (pneumonia at 4.86/100 PY, other acute lower respiratory infections at 5.45/100 PY). Age over 60 years and the presence of extra-articular manifestations were significantly associated with a higher risk of infections (Age, HR: 2.133; 95% CI: 1.118–4.071, P=0.022; extra-articular manifestations, HR: 14.566, 95% CI: 6.317-33.584, P< 0.0001). However, grade 3/4 neutropenia induced by tocilizumab administration was not associated with increased infection risk (HR: 0.721; 95% CI: 0.105-2.117; P=0.471).
Conclusion: Approximately 7% of patients with RA treated with tocilizumab developed grade 3/4 neutropenia. A previous history of neutropenia due to csDMARD therapy was an important predictor for the onset of tocilizumab-induced neutropenia. Age and extra-articular manifestations, but not neutropenia, were associated with infection, indicating that tocilizumab-induced neutropenia was not clinically significant in the incidence of infection in patients with RA.
To cite this abstract in AMA style:
Kim Y, Ahn S, Oh J, Kim Y, Lee C, Yoo B, Hong S. Retrospective Analysis of Tocilizumab-induced Neutropenia in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/retrospective-analysis-of-tocilizumab-induced-neutropenia-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retrospective-analysis-of-tocilizumab-induced-neutropenia-in-patients-with-rheumatoid-arthritis/