Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite the beneficial effects of hydroxychloroquine (HCQ) in systemic lupus erythematosus (SLE), retinal toxicity is a concern. Factors associated with retinal toxicity have been studied among long-term HCQ users but have not been described for incident SLE patients. We evaluated the incidence of HCQ-related retinal toxicity in a large, international, inception cohort of SLE patients, and assessed factors potentially associated with this event.
Methods: We analyzed prospective data from the Systemic Lupus International Collaborating Clinics (SLICC) cohort, that includes SLE patients from 33 sites in Europe, Asia, and North America, enrolled within 15 months of diagnosis. Using annual study visits between 1999-2019, we followed patients from first visit on HCQ (time zero/baseline) up to the time of retinal toxicity documentation (outcome) or death, loss to follow-up, or censoring at end of study interval. Retinal toxicity was identified based on the SLICC/ACR damage index item for retinal damage and cases were confirmed with chart review. Multivariable Cox regression was used to estimate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for baseline factors potentially associated with retinal toxicity (i.e., sex, race/ethnicity, age at SLE onset, HCQ daily dose/kg, body mass index, and smoking). We also plotted a Kaplan-Meier curve for probability of retinal toxicity related to total duration of HCQ therapy.
Results: A total of 1460 patients (89% female, 52% Caucasian) were included. Mean SLE duration at time zero (HCQ initiation) was 2.4 (standard deviation, SD 2.2) years and patients remained on HCQ an average of 6.4 (SD 4.2) years. Retinal toxicity was confirmed for 11 patients (incidence 1.0 per 1000 person-years) at a mean of 8.8 (SD 4.0) years. Our hazards regression model (Table 1) identified non-significant trends for greater risk in men, black patients, those receiving more than 5 mg per kg at baseline, overweight patients and smokers. In the Kaplan-Meier curve (Figure 1), the crude probability of retinal toxicity was less than 1% until 10 years of cumulative HCQ use, but increased around 1% each year after that, reaching 5% after 14 years.
Conclusion: In recent-onset SLE patients receiving HCQ, the probability of retinal toxicity increases after 10 years of cumulative use. There were non-significant trends for greater risk in men, black patients, those receiving more than 5 mg per kg at baseline, overweight patients and smokers. More sophisticated analyses with time-dependent variables are under way.
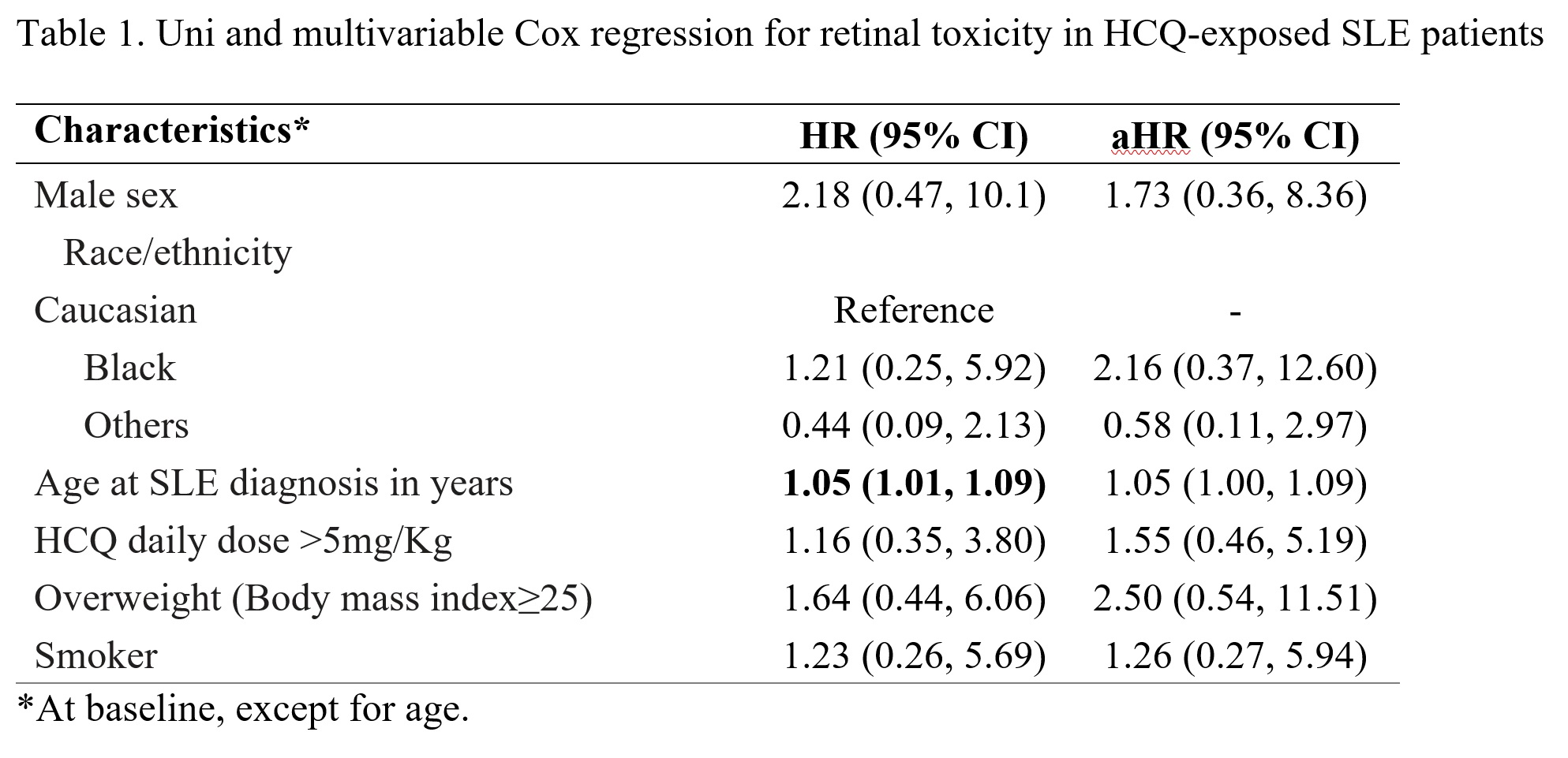 Table 1. Uni and multivariable Cox regression for retinal toxicity in HCQ-exposed SLE patients
Table 1. Uni and multivariable Cox regression for retinal toxicity in HCQ-exposed SLE patients
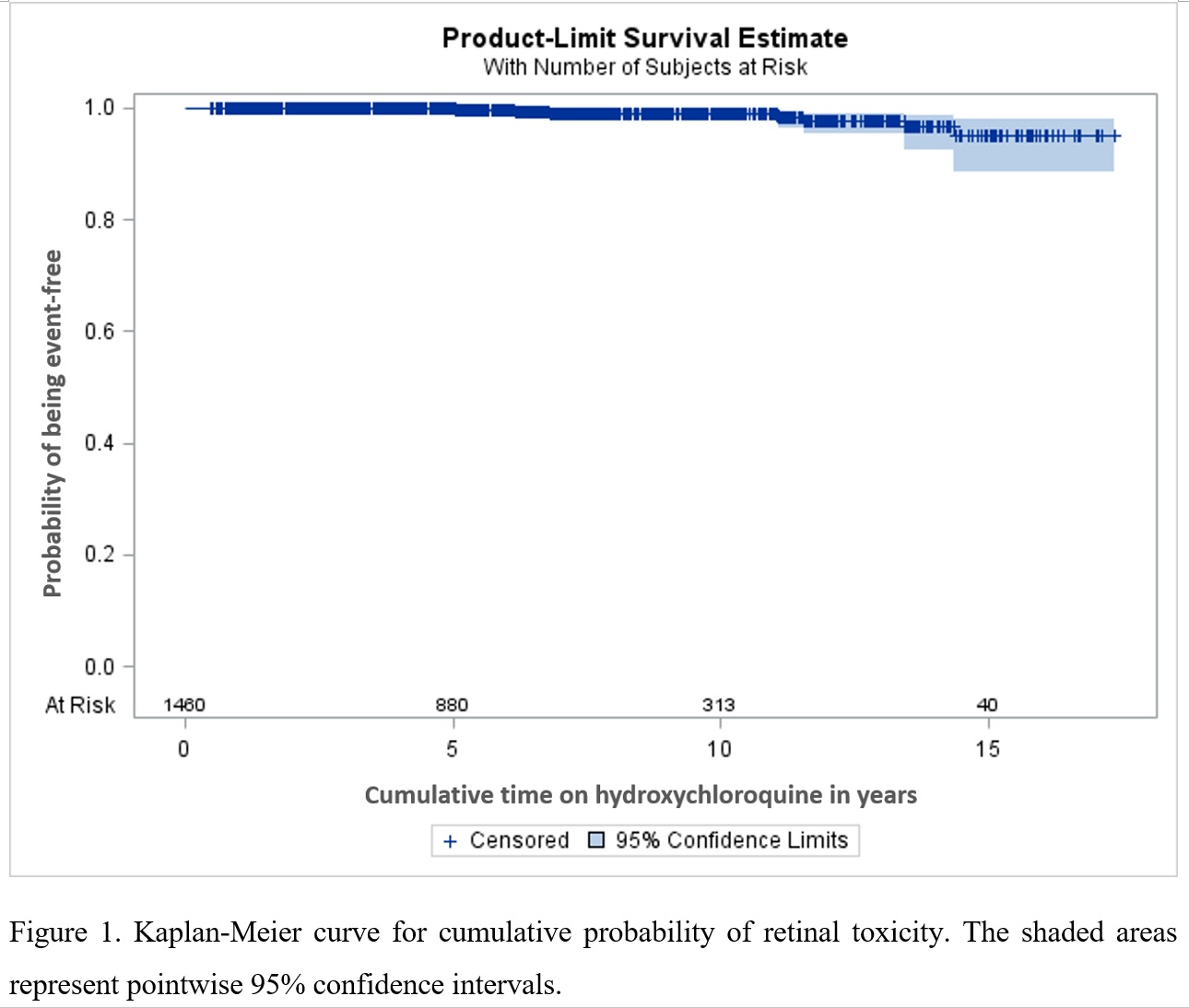 Figure 1. Kaplan-Meier curve for cumulative probability of retinal toxicity. The shaded areas represent pointwise 95% confidence intervals.
Figure 1. Kaplan-Meier curve for cumulative probability of retinal toxicity. The shaded areas represent pointwise 95% confidence intervals.
To cite this abstract in AMA style:
Almeida-Brasil C, Hanly J, Urowitz M, Clarke A, Ramsey-Goldman R, Gordon C, Petri M, Ginzler E, Wallace D, Bae S, Romero-Díaz J, Dooley M, A. Peschken C, Isenberg D, Rahman A, Manzi S, Jacobsen S, Lim S, Van Vollenhoven R, Nived O, Jönsen A, Kamen D, Aranow C, Ruiz-Irastorza G, Sanchez-Guerrero J, Gladman D, Fortin P, Alarcón G, Merrill J, Kalunian K, Ramos-Casals M, Steinsson K, Zoma A, Askanase A, Khamashta M, Bruce I, Inanc M, Bernatsky S. Retinal Toxicity in a Multinational Inception Cohort of Systemic Lupus Patients on Hydroxychloroquine [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/retinal-toxicity-in-a-multinational-inception-cohort-of-systemic-lupus-patients-on-hydroxychloroquine/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retinal-toxicity-in-a-multinational-inception-cohort-of-systemic-lupus-patients-on-hydroxychloroquine/
