Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Erdheim-Chester disease (ECD) is a rare non-Langerhans histiocytosis with a broad clinical spectrum. The therapeutic landscape of ECD has remarkably changed over the last decade following the identification of activating mutations of the MAPK-ERK pathway. Patients harboring the BRAF V600E mutation can be treated with small molecules targeting BRAF (e.g., vemurafenib and dabrafenib). MEK inhibitors (e.g., cobimetinib and trametinib) are effective in patients with other activating mutations or those refractory or intolerant to BRAF inhibitors. Though effective, these medications are associated with a significant toxicity burden. The interleukin 1 receptor antagonist anakinra has been shown to potentially decrease such adverse reactions. Retention rates of these targeted drugs (DRRs) have not yet been reported in patients with ECD.
Methods: ECD patients from two Italian referral Centres who received at least one targeted therapy were retrospectively identified. Data regarding demographics, disease characteristics, outcome, and treatment were collected. Number of targeted agents, reasons for discontinuation and previous and concomitant therapies were analyzed. DRRs at 18 months were calculated for each targeted drug. Survival curves were obtained with the Kaplan-Meier method and compared using a stratified log-rank test. Hazard ratio (HR) for concomitant anakinra treatment was evaluated.
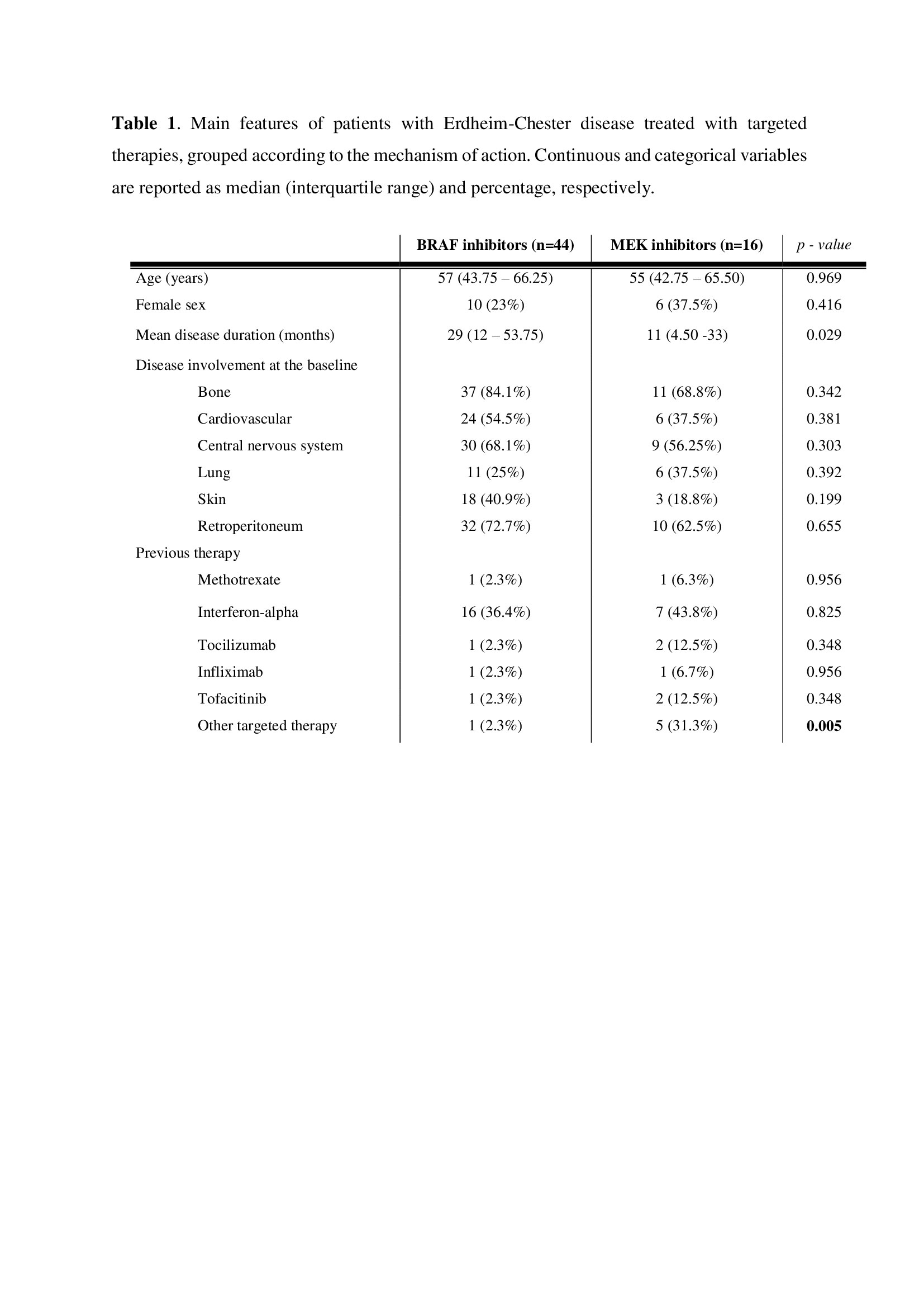
Results: Fifty-two patients with ECD were treated with at least one targeted therapy, accounting for a total of 60 treatment courses. Demographics, disease features and treatment are shown in Table 1. Vemurafenib was the most used targeted medication (n=43 [72%]), followed by cobimetinib (n=14 [23%]), trametinib (n=2 [3%]), and dabrafenib (n=1 [2%]). The overall DRR at 18 months was 70%. DRRs did not statistically differ among vemurafenib and cobimetinib (72% versus 64%), survival curves shown in Figure 1. Trametinib and dabrafenib were not suspended throughout the entire period of observation. The most common reason of discontinuation was adverse reaction (n=13 [76%] cases). Seventeen (28%) patients received an upfront combined treatment with anakinra. As shown in Figure 1, this combination approach was associated with a significantly higher DRR (94% vs 65%, p = 0.040). Anakinra concomitant had a positive effect on DRR (HR=1.46, 95% CI=1.15-2.74)
Conclusion: In ECD patients treated with targeted therapies, the overall DRR at 18 months was over 70% and there was no difference according to the specific molecule. Combination treatment with anakinra resulted in significantly higher drug persistence.
To cite this abstract in AMA style:
Campochiaro C, tomelleri a, Pegoraro F, Galardi G, Farina N, Mazzariol M, Catamerò F, De Luca G, Cavalli G, Vaglio A, Dagna L. Retention Rate of Targeted Therapies in Erdheim-Chester Disease [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/retention-rate-of-targeted-therapies-in-erdheim-chester-disease/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/retention-rate-of-targeted-therapies-in-erdheim-chester-disease/