Session Information
Date: Tuesday, November 14, 2023
Title: (1945–1972) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient reported outcome measures (PROMs) are critical in assessing clinical outcomes. There is a paucity of PROMs for use in patients with adult idiopathic inflammatory myopathies (IIM). The Outcome Measures in Rheumatology (OMERACT) Myositis Working Group was established to develop validated PROMs for adult IIM. Our previous work has included identifying the domains of interest as pain interference, fatigue, and physical function and demonstrating the content validity, test-retest reliability, and construct validity of the PROMIS pain interference (6a), fatigue (7a), and physical function (8b) instruments. In this study, our goal was to assess the responsiveness and minimal important difference of these instruments in a prospective longitudinal cohort of adults with IIM.
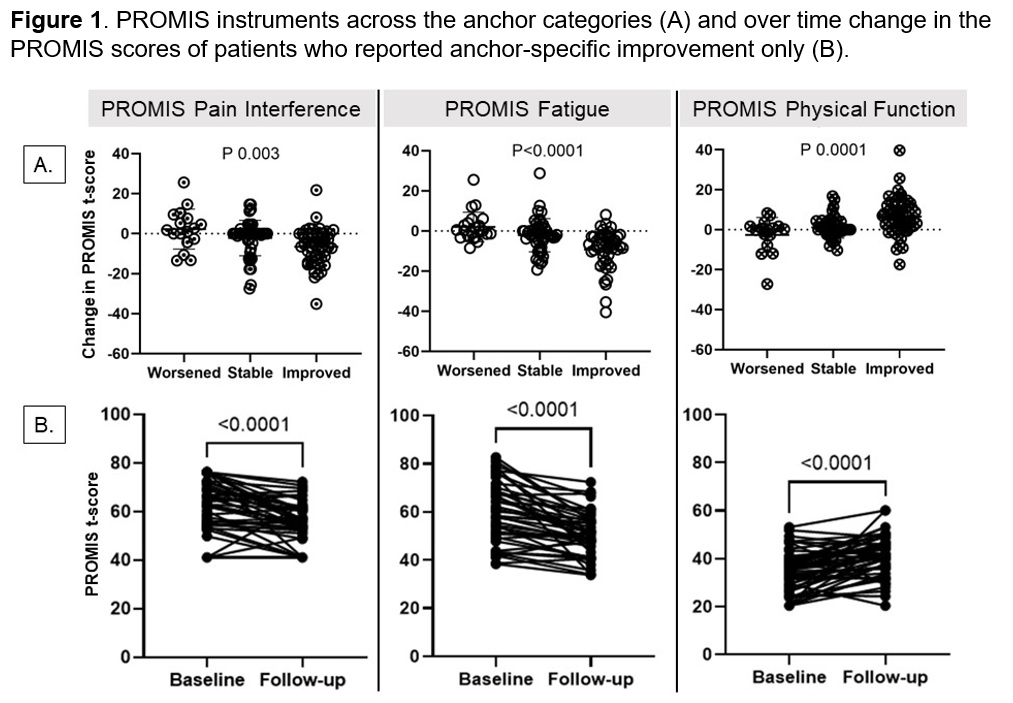
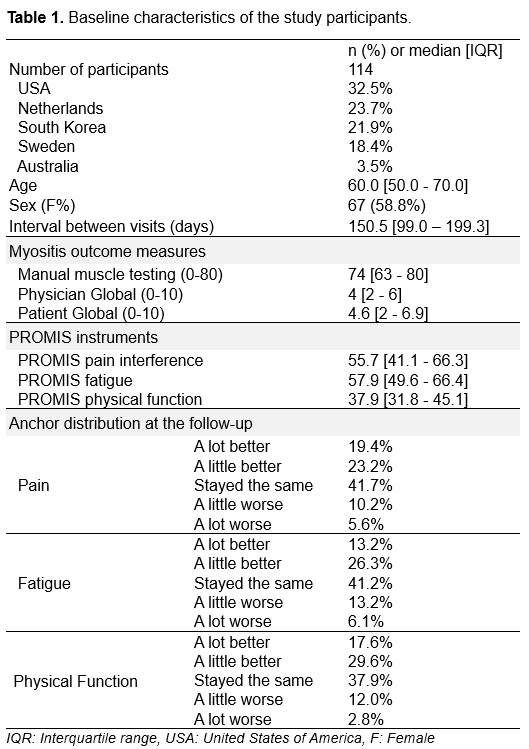
Methods: Adults with IIM who were seen in the outpatient clinics in United States of America (USA), Netherlands, South Korea, Sweden, and Australia, were enrolled in this prospective observational study with two study visits. Relevant myositis core set measures (manual muscle testing [MMT8, 0-80], physician and patient global disease activity [0-10]), and the PROMIS instruments for pain interference (6a), fatigue (7a), and physical function (8b) were collected at the baseline and follow-up visits. Domain-specific anchor questions for symptoms of pain, fatigue, and physical function were asked at the follow-up visit (a lot better, a little better, stable, a little worse, a lot worse). Responsiveness of the PROMIS instruments was assessed using i) ANOVA to compare the scores across the anchor categories (worsened, stable, improved), ii) paired t test, effect size and standardized response mean for within-person score change, and iii) Pearson correlation with myositis outcome measures. Effect size was interpreted as small for < 0.2, medium for 0.2-0.5, and large for >0.5. Minimal important difference was calculated using the anchor-based method.
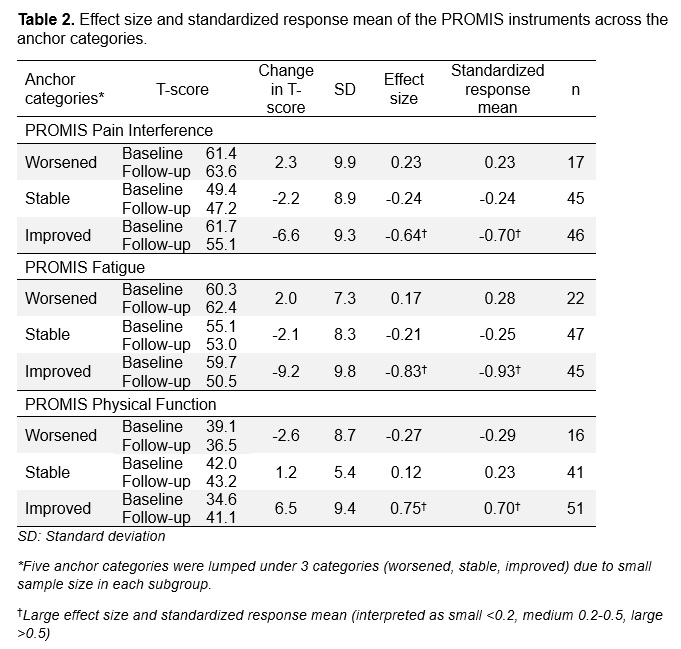
Results: 114 patients with IIM (median age of 60 [50-70], 60% female) completed two study visits at five international sites (USA [32.5%], Netherlands [23.7%], Korea [21.9%], Sweden [18.4%], Australia [3.5%])(Table 1). Changes in PROMIS pain interference, fatigue, and physical function were significantly different among anchor categories (Figure 1). Patients who reported improvement in domain-specific anchor questions had a significant improvement in their PROMIS scores with at least medium effect size ( >0.5), while patients who reported worsening and stability in anchor questions did not show a significant change with weak effect size (< 0.2) (Table 2). PROMIS instruments had weak to moderate correlations with MMT, patient and physician global. Using the anchor-based method, the minimal important difference was 2.7 t-score for PROMIS pain interference, 4 for fatigue, and 3.3 for physical function.
Conclusion: This study provides evidence towards the responsiveness of the PROMIS instruments in a large international prospective cohort of adults with IIM supporting their use as PROMs in adult myositis. Further work is planned to examine responsiveness in a larger cohort of those who worsen and in clinical trials.
To cite this abstract in AMA style:
Saygin D, Direnzo D, Raaphorst J, Park J, de Groot I, Bingham C, Lundberg I, Regardt M, Sarver c, de Visser M, maxwell l, Beaton D, Kim J, Needham M, Alexanderson H, Christopher-Stine L, Mecoli C. Responsiveness and Minimal Important Difference of PROMIS Pain Interference, Fatigue, and Physical Function Forms in Adults with Idiopathic Inflammatory Myopathies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/responsiveness-and-minimal-important-difference-of-promis-pain-interference-fatigue-and-physical-function-forms-in-adults-with-idiopathic-inflammatory-myopathies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/responsiveness-and-minimal-important-difference-of-promis-pain-interference-fatigue-and-physical-function-forms-in-adults-with-idiopathic-inflammatory-myopathies/