Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept, a selective T-cell co-stimulation modulator, has been demonstrated to be well tolerated and effective in JIA in 2 Phase III studies.1,2 The ongoing Phase IV Pediatric Rheumatology Collaborative Study Group and Paediatric Rheumatology International Trial Organization (PRCSG/PRINTO) registry aims to provide monitoring data from a real-world setting regarding longitudinal effectiveness and safety of abatacept in JIA. Here we assess the effectiveness of abatacept in JIA categories in patients who enrolled ≤1 month after starting abatacept treatment.
Methods: Using a standardized protocol and data collection process, clinical sites enroll patients with JIA currently receiving/starting IV/SC abatacept and follow them for up to 10 years. Patients are assessed at baseline and at 3, 6, 9, 12, and 24 months. Disease-related quality of life and physical function were quantified using the Juvenile Arthritis Multidimensional Assessment Report scale.3 Proportions of patients with JIA-ACR 30, 50, 70, and 90 level of responses were determined using validated definitions based on 5/6 JIA core set measures (CRP/ESR not included).4 The clinical 10-joint Juvenile Arthritis Disease Activity Score (cJADAS10) used validated cut-offs for low disease (LD) and inactive disease (ID) activity.5 Disease remission was defined as cJADAS10-validated ID for ≥6 months. As-observed analysis is presented.
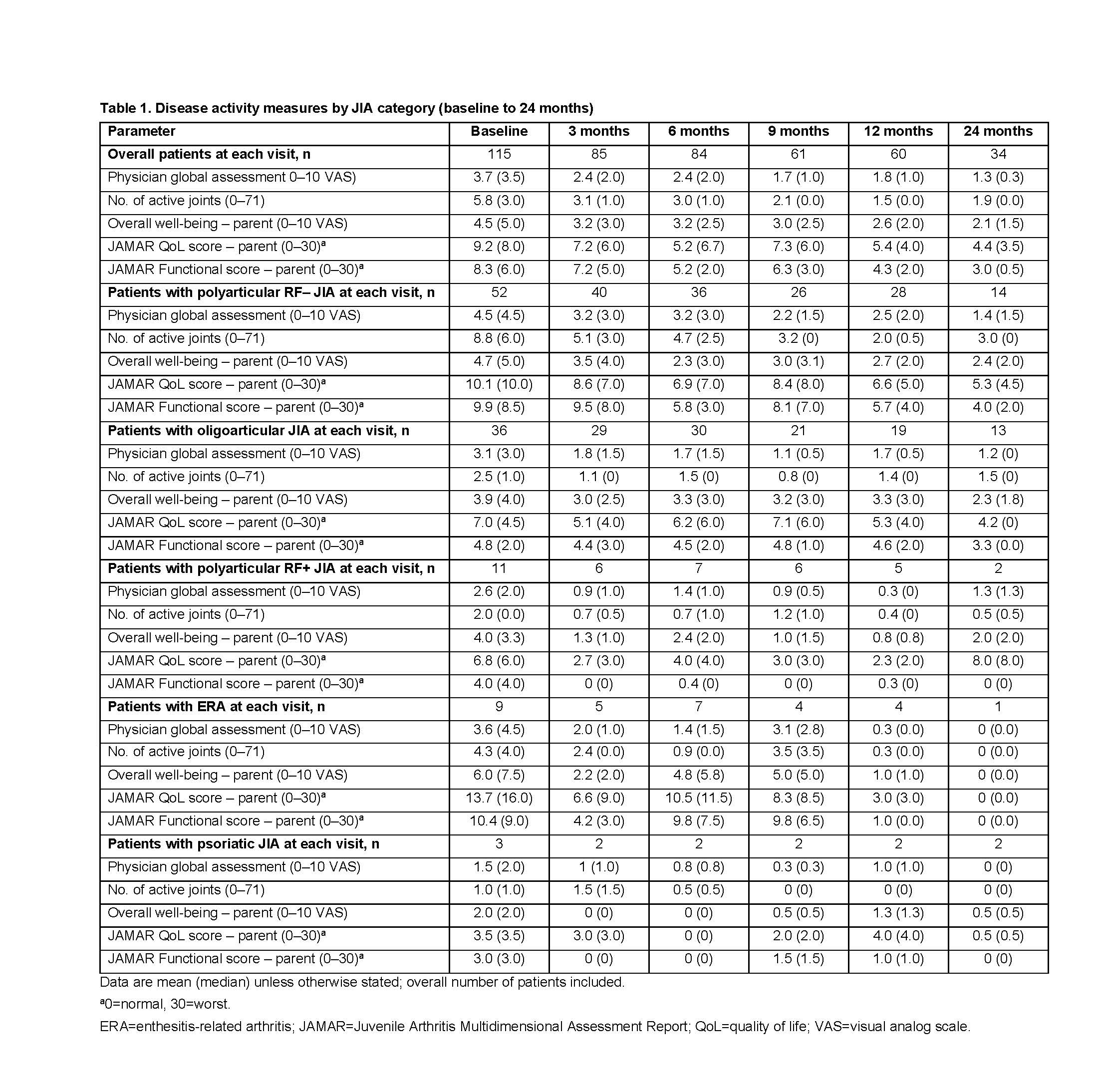
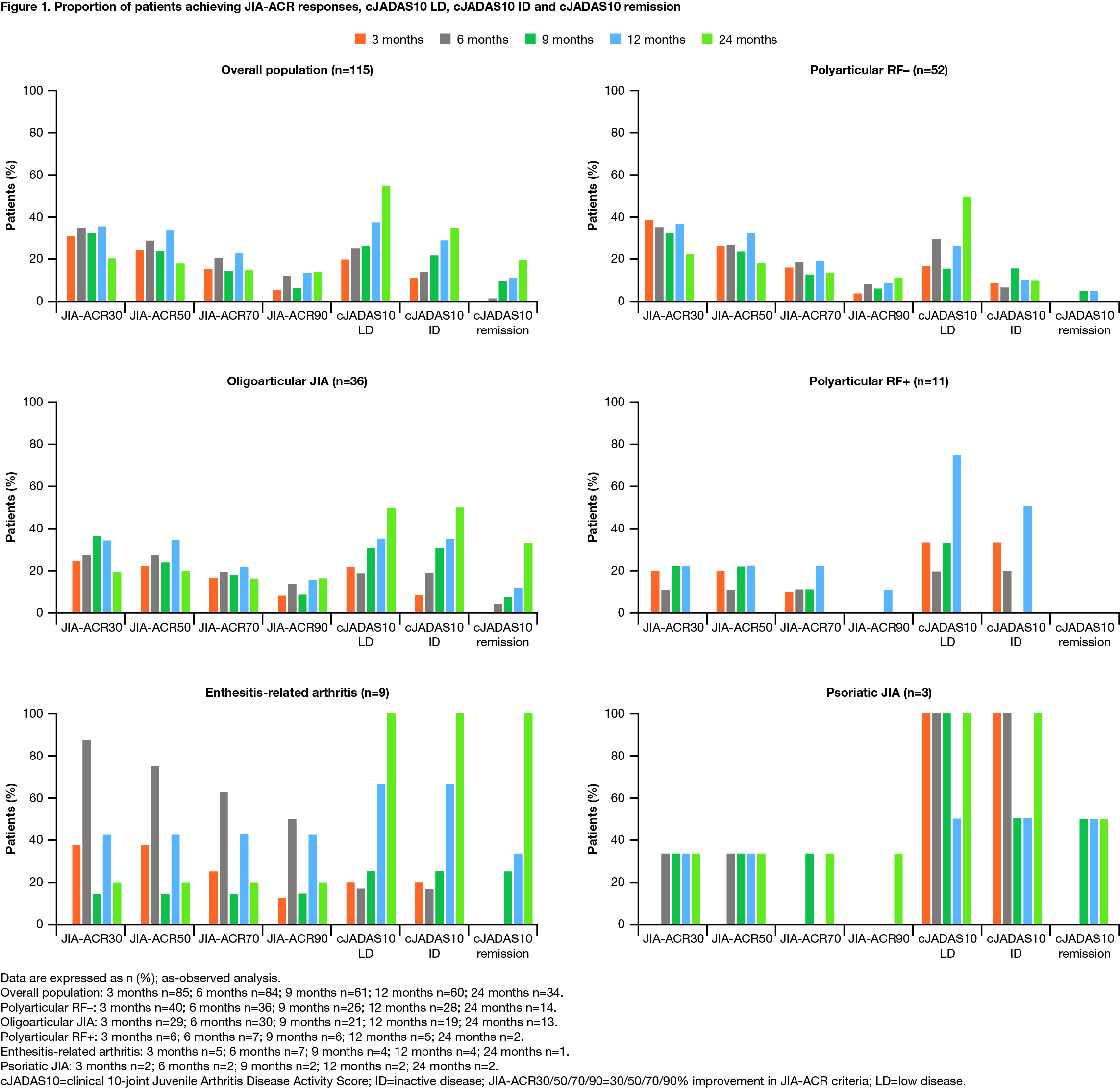
Results: As of March 31, 2018, 115 patients were included. Of these, 93 (80.9%) were female, the baseline mean (median) age at enrollment was 12.8 (13.1) years, and disease duration was 60.0 (42.1) months. The JIA categories identified were: polyarticular RF–, 52 (45.2%); oligoarticular, 36 (31.3%); polyarticular RF+, 11 (9.6%); enthesitis-related arthritis (ERA), 9 (7.8%); psoriatic and undifferentiated, 3 (2.6%) each; systemic, 1 (< 1%) (Table 1; patient with systemic JIA excluded). The proportions of patients achieving JIA-ACR responses, cJADAS10 LD, cJADAS10 ID and cJADAS10 remission are shown in Figure 1 (patient with systemic JIA excluded).
Conclusion: Abatacept treatment resulted in rapid, clinically important and sustained JIA-ACR responses in all JIA categories with polyarticular or oligoarticular disease course and few achieved cJADAS10 ID and cJADAS10 remission status. Quality of life was also improved across JIA categories over time, as per JAMAR QoL scores. Limitations of the study include a low number of patients with ERA, psoriatic, undifferentiated, and systemic JIA.
References:
- Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–1154.
- Ruperto N, et al. Lancet 2008;372:383–391.
- Solari N, et al. Pediatr Rheumatol Online J 2008;6(Suppl 1):P106.
- Giannini EH, et al. Arthritis Rheum 1997;40:1202–1209.
- Consolaro A, et al. Arthritis Care Res (Hoboken) 2014;66:1703–1709.
Medical writing: Katerina Kumpan, PhD (Caudex).
To cite this abstract in AMA style:
Lovell D, Tzaribachev N, Morgan E, Simonini G, Griffin T, Alexeeva E, Bohnsack J, Zeft A, Horneff G, Vehe R, Stanevicha V, Tarvin S, Trachana M, Huber A, Kietz D, Orban I, Dare J, Foeldvari I, Quartier P, Dominique A, Kou T, Wong R, Martini A, Brunner H, Ruperto N. Response to Abatacept in JIA Categories: Results from the PRCSG/PRINTO JIA Abatacept Phase IV Registry [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/response-to-abatacept-in-jia-categories-results-from-the-prcsg-printo-jia-abatacept-phase-iv-registry/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/response-to-abatacept-in-jia-categories-results-from-the-prcsg-printo-jia-abatacept-phase-iv-registry/