Session Information
Date: Tuesday, November 12, 2019
Title: Orthopedics, Low Back Pain, & Rehabilitation Poster – ACR/ARP
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: CONSORT guidelines recommend the reporting of adverse events (AEs) and drop outs, along with a description of each. However, the application of these guidelines in clinical trials of exercise is unknown. This study aimed to identify how AEs and drop outs are reported in clinical trials of therapeutic exercise for adults with KOA.
Methods: Cochrane Library, EMBASE, MEDLINE, and CINAHL databases were searched to identify randomized controlled trials of therapeutic exercise for patients with KOA published in English from January 1, 1980 through December 31, 2016. The American Physical Therapy Association operational definition of therapeutic exercise was used. Databases were searched using the following search terms: knee osteoarthritis AND exercise OR exercise therapy OR strengthening OR aerobics OR anaerobic OR dynamic OR isotonic OR isometric OR isokinetic OR strengthening OR aquatic OR physical therapy AND clinical trial NOT modalities. Studies which compared non-pharmacologic interventions to therapeutic exercise were eligible as long as the therapeutic exercise intervention arm included only therapeutic exercise. Studies which included patients with other forms of arthritis or with non-specified knee pain were excluded. We focused on studies which reported outcomes related to impairments, functional limitations and participation. Four researchers independently examined each study and eliminated studies which did not meet our inclusion criteria. The reference list for each chosen article was also screened for eligible studies. In instances where the eligibility of the study was unclear, the researchers deliberated and came to a consensus regarding inclusion. Using a standardized form, we extracted information regarding participant and intervention characteristics, whether there was a clear statement of AEs and drop outs and reasons for AEs and drop outs. Study quality was assessed using the PEDRO scoring method and descriptive and inferential statistics were used to characterize results.
Results: One hundred two studies from 21 countries met the inclusion criteria with 6,654 subjects exercising. Of these 102 studies, 32 studies (31%) included a statement of AEs and 14 of these studies (44%) reported AEs. Eighty-five studies (83%) had a clear statement regarding drop outs and 20 studies (23%) gave reasons for drop out that could be classified as AEs. Forty percent of studies published in North America and Western Europe had a statement of AEs compared to 25% of studies from the rest of the world (p=0.13). Adverse events were reported for 59 patients and 55 individuals dropped out for reasons that could be considered an AEs, yielding 114 patients (2%) experiencing an AE-related to exercise.
Conclusion: Overall the quality of included studies was good. Geographic variations existed in statements about AEs. AEs and drop outs may be underreported and not sufficiently represent the risk associated with therapeutic exercise. Greater clarity regarding definitions of AEs and adherence to the CONSORT reporting guidelines are needed to best determine safe dosing of therapeutic exercise for KOA.
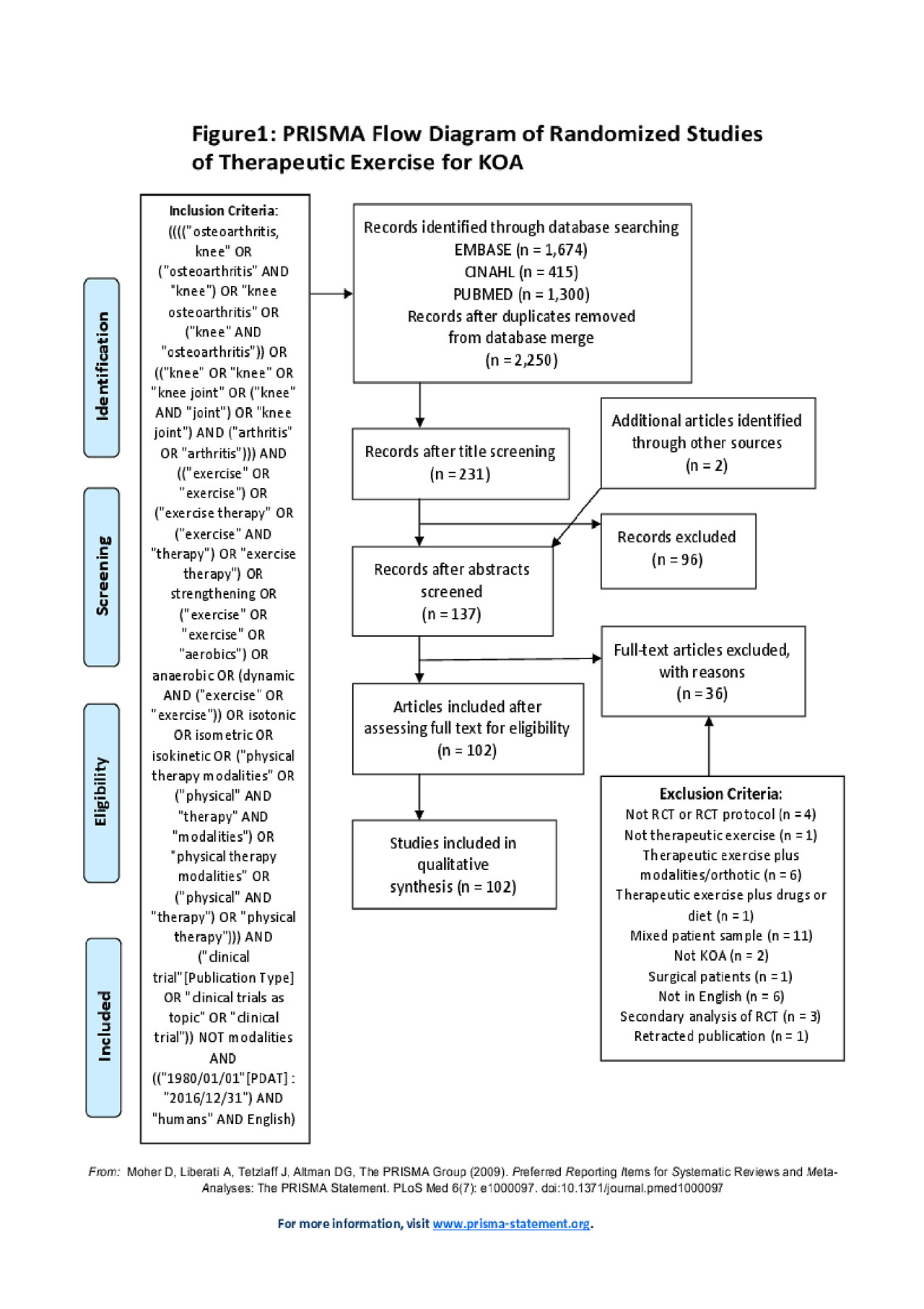
Updated- PRIMSA flow figure-5.24.19
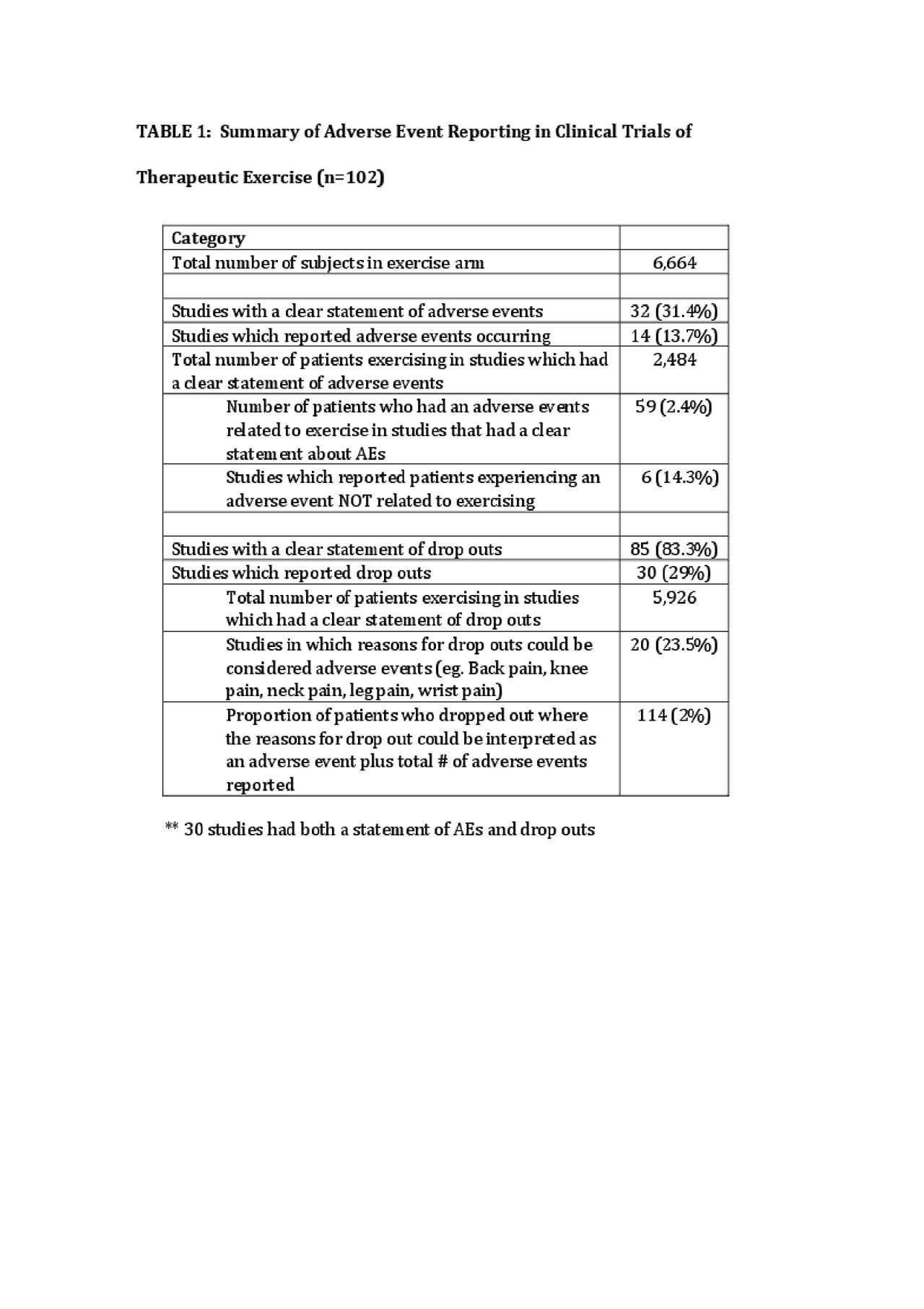
ACRabstractTables.Summary of AE Reporting in Clinical Trials
To cite this abstract in AMA style:
vonHeideken J, Iversen M. Reporting of Adverse Events in Randomized Controlled Trials of Therapeutic Exercise for Knee Osteoarthritis: A Systematic Review [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/reporting-of-adverse-events-in-randomized-controlled-trials-of-therapeutic-exercise-for-knee-osteoarthritis-a-systematic-review/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reporting-of-adverse-events-in-randomized-controlled-trials-of-therapeutic-exercise-for-knee-osteoarthritis-a-systematic-review/
