Session Information
Date: Sunday, November 12, 2023
Title: (0673–0690) Vasculitis – ANCA-Associated Poster I: Treatment Outcomes
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
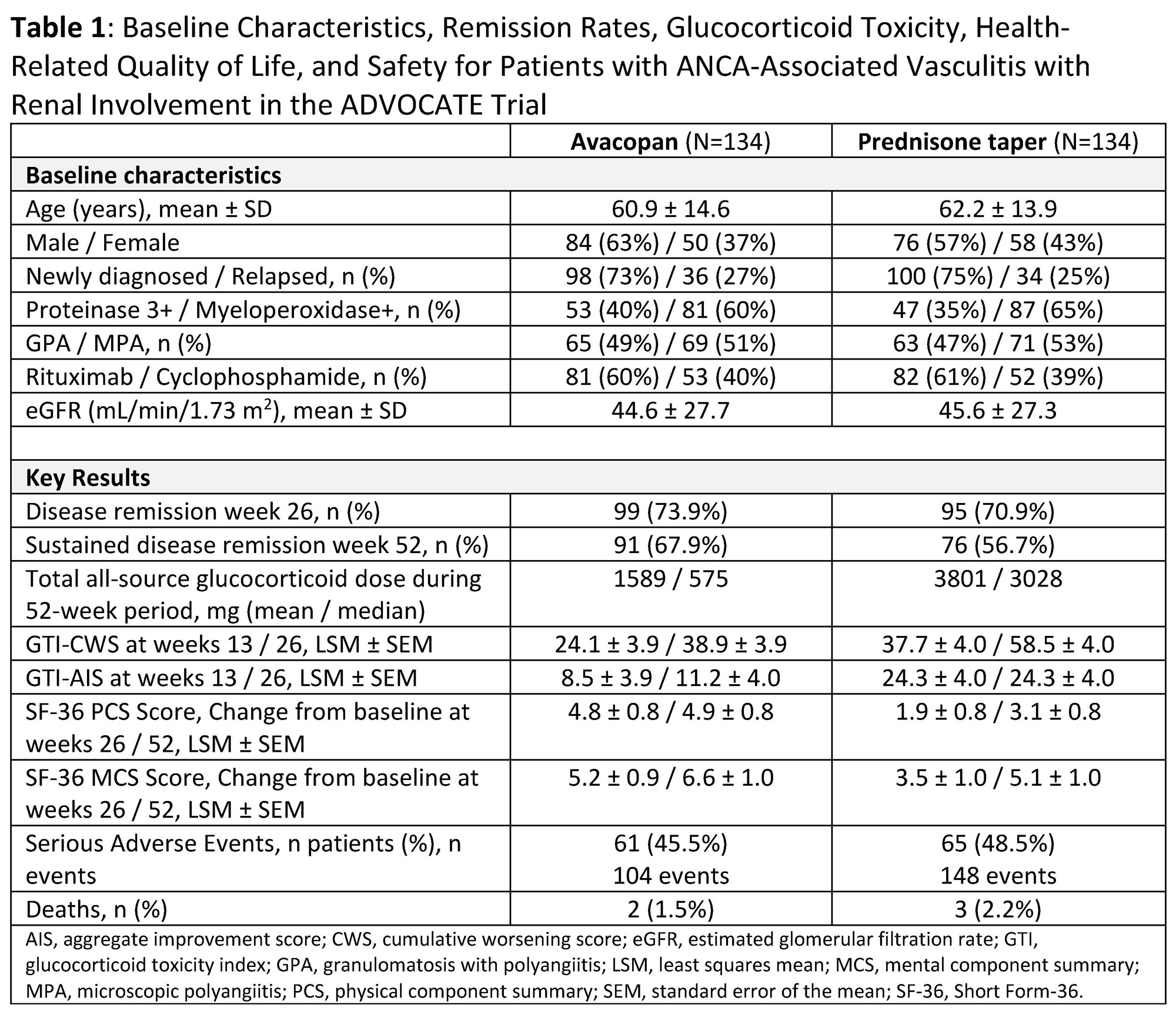
Background/Purpose: In the Phase 3 ADVOCATE trial comparing avacopan to a prednisone taper, 81% of patients with ANCA-associated vasculitis (AAV) had renal involvement based on the Birmingham Vasculitis Activity Score. This renal subgroup had a baseline mean estimated glomerular filtration rate of 45.1 mL/min/1.73 m2.
Methods: This post hoc analysis evaluated remission, glucocorticoid (GC) use, GC toxicity index (GTI), health-related quality of life (HRQoL by SF-36), and safety in patients with baseline renal involvement for those treated with avacopan (N=134) versus a prednisone taper (N=134).
Results: Compared to the overall study population, for this subgroup the mean age was similar (62 vs 61 years), but there was a slightly higher proportion of patients with newly diagnosed AAV (74% vs 69%), myeloperoxidase+ ANCA (63% vs 57%), microscopic polyangiitis (52% vs 45%), and use of cyclophosphamide (39% vs 35%). The avacopan group achieved a higher sustained remission rate at week 52 (67.9% vs 56.7%) while receiving a (mean/median) 2.4-/5.3-fold less total GC dose than the prednisone taper group (Table 1). The GTI cumulative worsening and aggregate improvement scores were lower at weeks 13 and 26 in the avacopan group compared to the prednisone group. At weeks 26 and 52 the avacopan group reported a greater improvement in SF-36 physical and mental component summary scores. Serious adverse events occurred in 46% (2 deaths) and 49% (3 deaths) of patients in the avacopan and prednisone groups, respectively.
Conclusion: In the ADVOCATE trial, patients with AAV with baseline renal involvement treated with avacopan achieved higher sustained remission rates while receiving less GCs, experiencing less GC-related toxicity, and reporting greater improvements in HRQoL versus those treated with a prednisone taper.
To cite this abstract in AMA style:
Geetha D, Cortazar F, Bruchfeld A, Karras a, Merkel P, Jayne D. Remission, Glucocorticoid Toxicity, Health-Related Quality of Life, and Safety Outcomes in Patients with Renal Involvement in the Phase 3 Trial of Avacopan for the Treatment of ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/remission-glucocorticoid-toxicity-health-related-quality-of-life-and-safety-outcomes-in-patients-with-renal-involvement-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculi/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remission-glucocorticoid-toxicity-health-related-quality-of-life-and-safety-outcomes-in-patients-with-renal-involvement-in-the-phase-3-trial-of-avacopan-for-the-treatment-of-anca-associated-vasculi/