Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with SLE, remission is an established therapeutic goal associated with reduced damage accrual and flares, and improved health-related quality of life.1 Here, we evaluated attainment of DORIS (Definition of Remission in SLE) during anifrolumab treatment in the double-blind randomized phase 3 TULIP long-term extension (LTE) trial.2
Methods: Patients with moderate to severe SLE (1997 ACR criteria) despite standard therapy who completed the 52-week TULIP-1 or TULIP-2 trials (NCT02446912, NCT02446899) could reconsent to participatein the randomized, placebo-controlled, double-blind, 3-year extension (NCT02794285). Here, we analyzed patients randomized to receive intravenous anifrolumab 300 mg or placebo for the TULIP-1/TULIP-2 and LTE periods. DORIS attainment was defined as all the following: total clinical SLEDAI-2K score =0, physician global assessment (0–3) < 0.5, prednisone/equivalent dosage ≤5 mg/day, and no use of restricted medications (TULIP-1/TULIP-2 period only). Time to first DORIS was compared using Cox regression. DORIS attainment rates were calculated using a Cochran–Mantel–Haenszel approach and compared using logistic regression. Total time and percentage of time in DORIS was analyzed using analysis of covariance. Patients who discontinuedinvestigational product prematurely and/or withdrew from the study due to lack of efficacy and/or disease worsening were considered nonresponders from that visit on. All P-values are nominal.
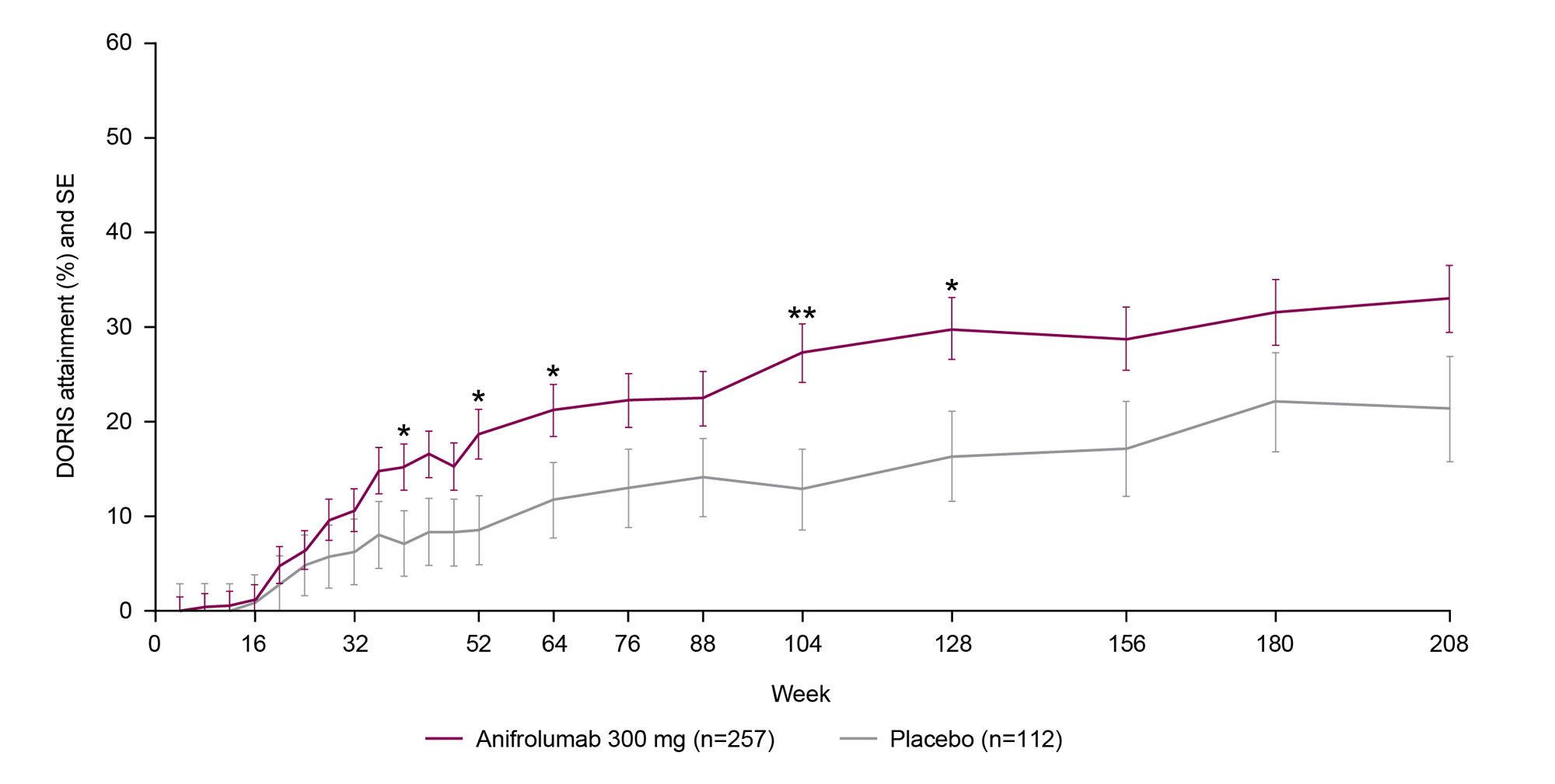
Results: Overall, 369 patients (anifrolumab 300 mg, n=257; placebo, n=112) who continued treatment in the LTE were analyzed for the 4-year TULIP+LTE period. Time to first DORIS remission was shorter in anifrolumab-treated patients compared with placebo (hazard ratio 1.49, 95% CI 1.04–2.19, P=0.034). DORIS remission attainment increased from TULIP baseline to Week 208; at the first LTE visit (Week 64), 21.2% of anifrolumab-treated patients achieved DORIS compared with 11.8% of the placebo group (odds ratio [OR] 2.1, 95% CI 1.0–4.1, P=0.036); a similar trend was seen up to Week 208 (33.0% vs 21.4%; OR 1.8, 95% CI 0.9–3.5, P=0.089) (Figure). Greater cumulative time (P=0.002) and percentage of time (P=0.002) were spent in DORIS by patients receiving anifrolumab compared with placebo. A greater proportion of patients treated with anifrolumab were in DORIS ≥20% of the time compared with placebo (OR 2.9, 95% CI 1.6–5.2, P=0.001) and ≥50% of the time (OR 2.3, 95% CI 1.1–5.0, P=0.030). Compared with placebo, anifrolumab-treated patients were more likely to sustain DORIS for ≥3 consecutive visits (OR 2.1, 95% CI 1.2–3.6, P=0.013) and ≥5 visits (OR 2.3, 95% CI 1.1–4.8, P=0.022).
Conclusion: Treatment with anifrolumab, in addition to standard therapy, was associated with more frequent, prolonged, and sustained DORIS remission attainment compared with placebo during the 4-year TULIP+LTE period. Our results suggest that DORIS, a definition of remission associated with improved clinical outcomes,1 is an attainable therapeutic goal with long-term anifrolumab use.
References: 1.van Vollenhoven RF, et al. Lupus Sci Med. 2021;8:e000538.
2.Kalunian KC, et al. Arthritis Rheumatol. 2023;75:253–65.
DORIS attainment was defined as all the following: total clinical SLEDAI_2K score =0, physician global assessment (0–3) <0.5, prednisone/equivalent dosage ≤5 mg/day, and no use of restricted medications (TULIP_1 and TULIP_2 period only). Clinical SLEDAI_2K is a variant of the SLEDAI_2K, in which the serological descriptors (anti-dsDNA and complement) are omitted.
DORIS attainment rates were calculated using a stratified Cochran–Mantel–Haenszel approach, with stratification factors of SLEDAI_2K at screening, Day 1 glucocorticoid dosage, type I interferon gene signature at screening, and study; response rates were compared using logistic regression.
DORIS, Definition of Remission in SLE; LTE, long-term extension; SE, standard error.
Nominal P: *<0.05; **<0.01.
To cite this abstract in AMA style:
van Vollenhoven R, Morand E, Furie R, Kalunian K, Tummala R, Abreu G, Al-Mossawi H, Lindholm C. Remission Attainment in Patients with Systemic Lupus Erythematosus Treated with Anifrolumab Compared with Placebo over a 4-Year Period [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/remission-attainment-in-patients-with-systemic-lupus-erythematosus-treated-with-anifrolumab-compared-with-placebo-over-a-4-year-period/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/remission-attainment-in-patients-with-systemic-lupus-erythematosus-treated-with-anifrolumab-compared-with-placebo-over-a-4-year-period/