Session Information
Date: Monday, November 8, 2021
Title: Systemic Sclerosis & Related Disorders – Clinical Poster II (1364–1390)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Raynaud’s Phenomenon (RP) leading to repetitive ischaemia and reperfusion (IR) stress, is the first recognisable sign of systemic sclerosis (SSc). Although RP has been linked to SSc aetiology, direct observations substantiating this are scarce. High-mobility group box-1 (HMGB1) is a nuclear factor released by necrotic cells, of which serum levels may rise quickly after IR injury in other diseases. IR injury promotes interferon (IFN) inducible genes, which have been implicated in SSc pathophysiology. Since HMGB1 can signal through the receptor for advanced glycation endproducts (RAGE), we investigated whether an RP attack promotes release of HMGB1, leading to fibroblast activation and upregulation of IFN- inducible genes in a translational study.
Methods: A cold challenge was performed to simulate an RP attack in patients with SSc (N=10, age 56.2 ± 11.5 years), primary RP (N=10, age 47.8 ± 16.2, 8), and healthy controls (N=10, age 29.9 ± 2.7). We measured levels of HMGB1 and IFN gamma-induced Protein 10 (IP-10) before, 10 and 30 min after cold challenge in blood drawn from ipsilateral forearm. Digital perfusion was assessed by photoplethysmography. For studying in vitro effects of HMGB1, healthy human dermal fibroblasts were stimulated with HMGB1 or TGF-β1 as control. Inflammatory (Interleukin-6 [IL-6]) genes, profibrotic (type 1 collagen [Col1], α-smooth muscle actin [α-SMA]), connective tissue growth factor [CTGF]) genes, and IFN-inducible genes (IFN α-induced44L [IFI144L], Myxovirus resistance protein 1 [Mx1], Lymphocyte antigen 6 complex, locus E [LY6E];, and IP-10) were measured by RT-PCR. RAGE signalling was determined by preincubation with its inhibitor FPS-ZM1. Differentiation to myofibroblasts was assessed by staining of α-SMA. In an independent cohort, sera were obtained from 20 patients with SSc (age 50 (44–54) years, 13 female) and 20 age- and sex-matched healthy controls (53 (47–63), 13) to determine HMGB1 and IP-10 levels.
Results: During cold challenge, finger perfusion was reduced in SSc and recovered slower than in primary RP and healthy controls (fig. 1a). HMGB1 increased significantly 30 min after cold challenge in SSc compared to healthy controls, but not to primary RP (fig. 1b). IP-10 remained stable. In vitro stimulation of fibroblasts with HMGB1 resulted in 20-fold increase in mRNA expression of IP-10, while IL-6 increased 3-fold. Col1, α-SMA, IFI144L, Mx1, and LY6E did not change (fig 2a). IP-10 expression was inhibited by 50% by preincubation with FPS-ZM1 (fig 2b). TGF-β1 stimulation promoted IL-6 and CTGF, without effects on IFN gene expression. HMGB1 stimulation induced myofibroblast differentiation and formation of α-SMA fibers. Both HMGB1 and IP-10 were significantly higher in patients with SSc compared to healthy controls (fig 3).
Conclusion: In this translational study, we show for the first time that an RP attack in patients with SSc leads to release of HMGB1. In vitro, HMGB1 induces IFN regulated gene expression in fibroblasts, especially interferon-γ inducible IP-10, at least in part in a RAGE dependent manner. This potentially links HMGB1 release following an RP attack to IFN inducible proteins as putative sequential steps leading to disease progression in SSc.
 Figure 1: Cold challenge in patients with SSc, primary RP and healthy controls showing effects on fingers perfusion (fig. 1a). The hand is gradually cooled from 33 to 6 degrees Celsius within 36 minutes, and rewarmed at ambient temperature (22 degrees Celsius) to assesses recovery of perfusion. In fig. 1b the effect of the cold challenge is shown on levels of HMGB1. Fig. 1c shows that hand is submerged in water to perform the cold challenge, and digital perfusion is measured by photoplethysmography.
Figure 1: Cold challenge in patients with SSc, primary RP and healthy controls showing effects on fingers perfusion (fig. 1a). The hand is gradually cooled from 33 to 6 degrees Celsius within 36 minutes, and rewarmed at ambient temperature (22 degrees Celsius) to assesses recovery of perfusion. In fig. 1b the effect of the cold challenge is shown on levels of HMGB1. Fig. 1c shows that hand is submerged in water to perform the cold challenge, and digital perfusion is measured by photoplethysmography.
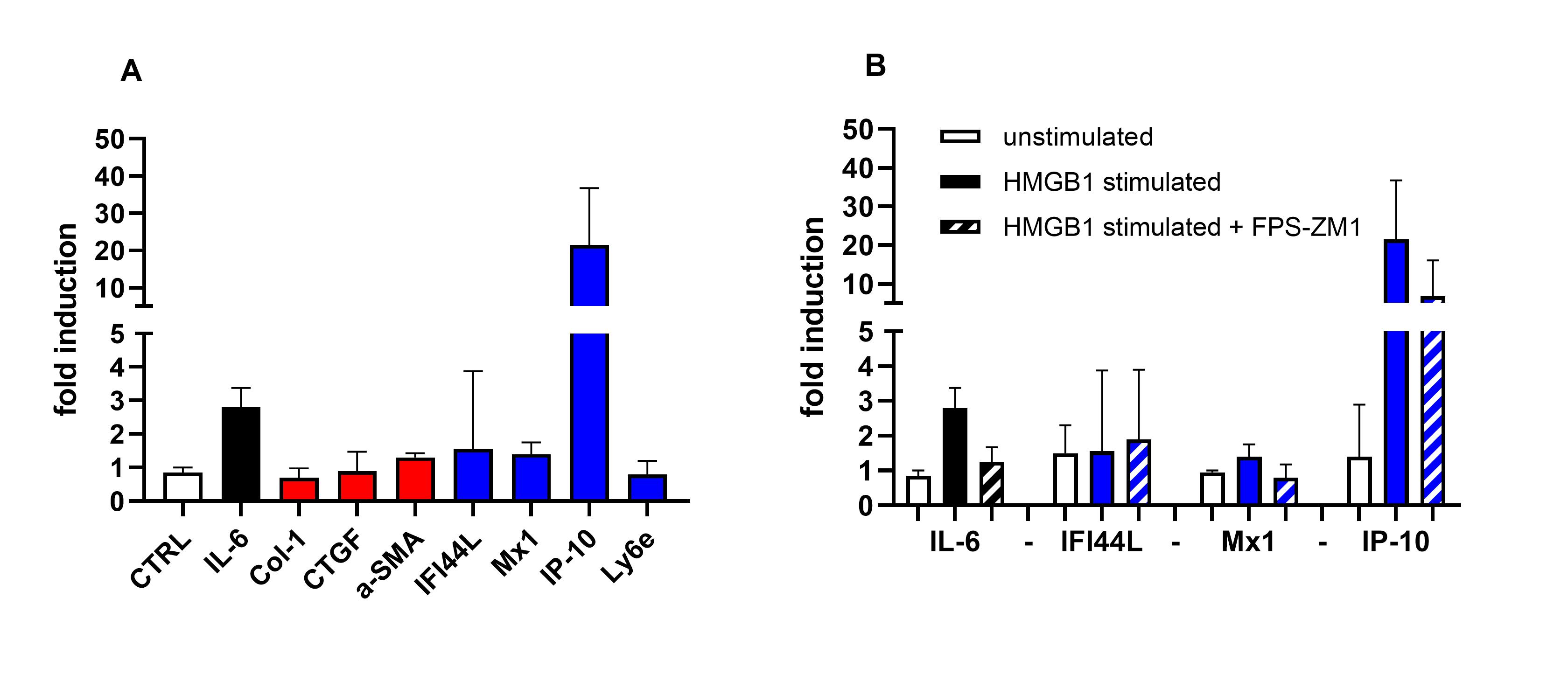 Figure 2: In vitro stimulation of dermal fibroblast with HMGB1. Fig. 2a shows induction of inflammatory (black), profibrotic (red), and IFN genes (blue), and negative control (white). Fig 2b shows the RAGE inhibitor FPS-ZM1 on IL-6 (black) and IFN genes (blue).
Figure 2: In vitro stimulation of dermal fibroblast with HMGB1. Fig. 2a shows induction of inflammatory (black), profibrotic (red), and IFN genes (blue), and negative control (white). Fig 2b shows the RAGE inhibitor FPS-ZM1 on IL-6 (black) and IFN genes (blue).
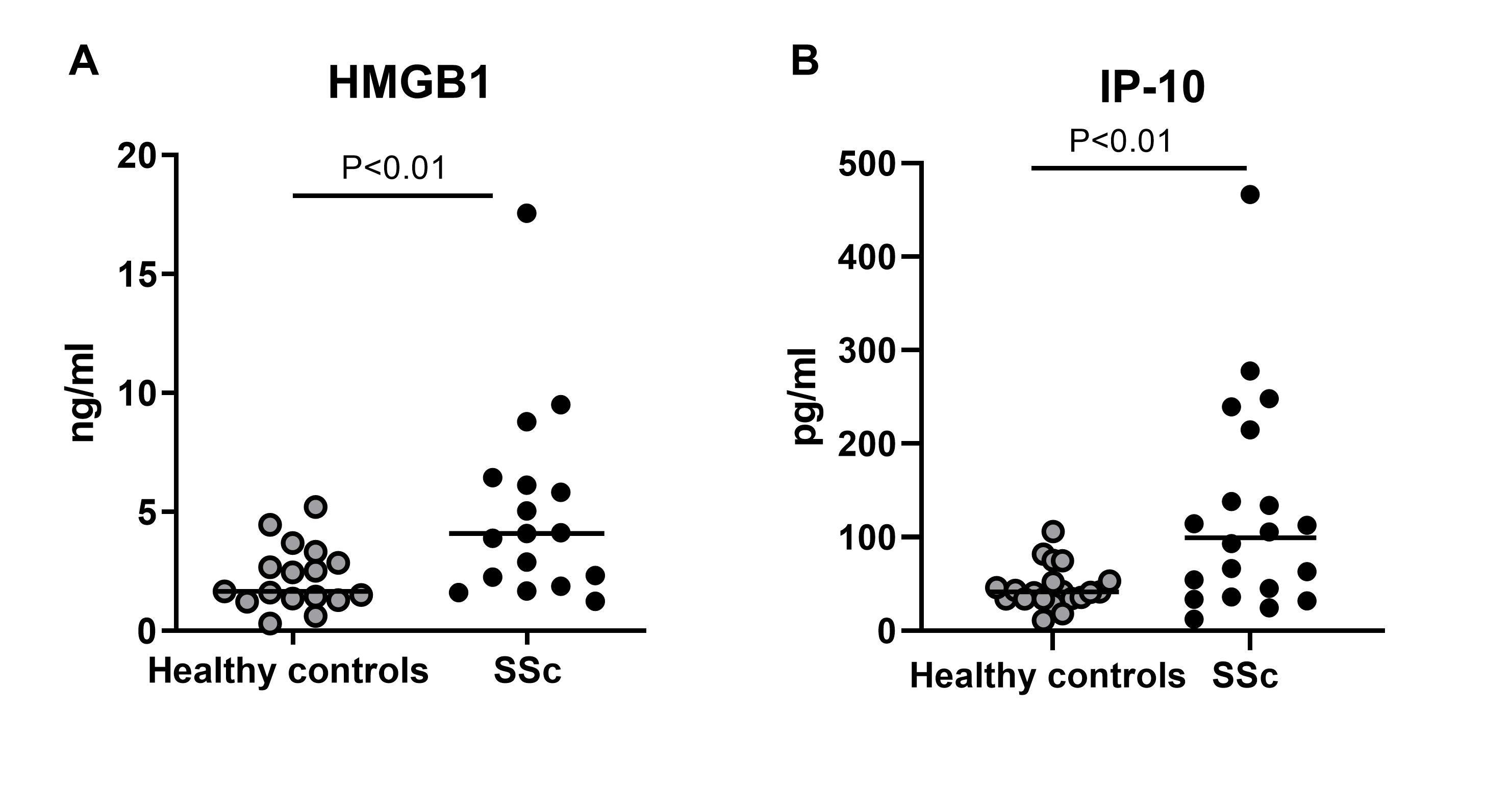 Figure 3: Serum levels of HMGB1 (fig. 3a) and IP_10 (fig. 3b) in patients with SSc compared with age and sex matched healthy controls.
Figure 3: Serum levels of HMGB1 (fig. 3a) and IP_10 (fig. 3b) in patients with SSc compared with age and sex matched healthy controls.
To cite this abstract in AMA style:
Atzeni I, Al-Adwi Y, Doornbos-van der Meer B, Eman Abdulle A, Van Roon A, Stel A, Van Goor H, Smit A, Westra J, Mulder D. Release of High-Mobility Group Box-1 After an Raynaud’s Attack Potentially Leads to Fibroblast Activation and Interferon-γ Induced Protein-10 Production in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/release-of-high-mobility-group-box-1-after-an-raynauds-attack-potentially-leads-to-fibroblast-activation-and-interferon-%ce%b3-induced-protein-10-production-in-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/release-of-high-mobility-group-box-1-after-an-raynauds-attack-potentially-leads-to-fibroblast-activation-and-interferon-%ce%b3-induced-protein-10-production-in-systemic-sclerosis/
