Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Presence of structural damage is an important risk factor for further damage progression over time in RA. Inhibition of damage progression is a key outcome measure in RA trials.
We assessed the effect of baseline (BL) structural damage, based on X-ray erosion scores (ES), on the progression of structural damage and clinical response over 52 weeks (wks) with subcutaneous (SC) abatacept (ABA) treatment in MTX-naïve, ACPA+ patients (pts) with early RA in the phase 3b AVERT-2 study (NCT02504268).1
Methods: Pts with early RA (ACR/EULAR 2010)2 were randomized to weekly SC ABA 125 mg or SC placebo (PBO), both with oral MTX, for 56 wks. X-rays were evaluated using modified total Sharp/van der Heijde score. This retrospective analysis included all pts with X-rays (BL and wk 52) and select clinical outcomes available.
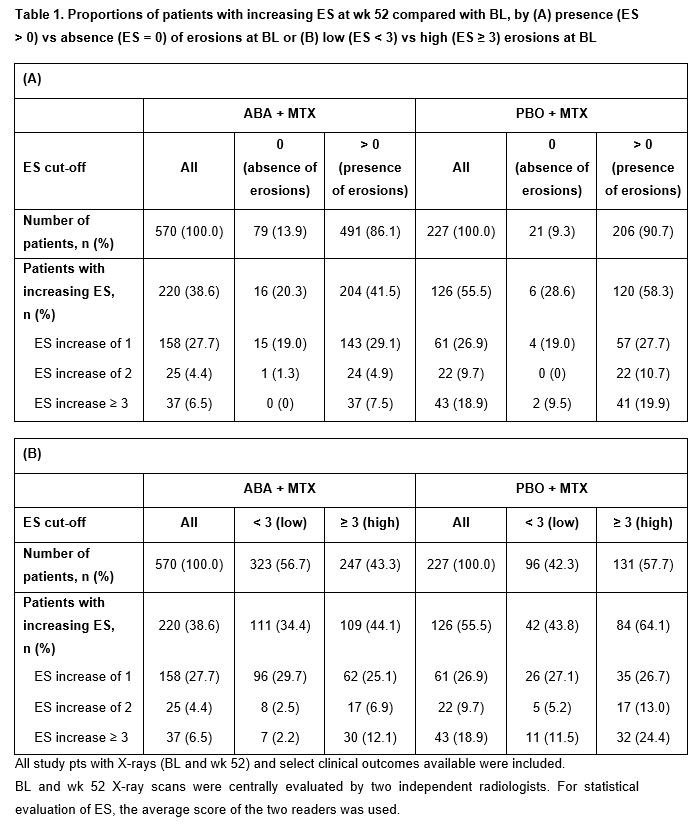
Comparisons were made between pts with BL structural damage presence (ES > 0) vs absence (ES = 0), or high (ES ≥ 3) vs low (ES < 3) structural damage, in either arm and between ABA + MTX vs PBO + MTX arms. Outcome measures at wk 52 included: proportions of pts with ES increase ≥ 1 and by subgroups (increases of 1, 2, ≥ 3); change from BL in ES; and clinical outcomes (Simplified Disease Activity Index [SDAI] remission [≤ 3.3], DAS28 [CRP] < 2.6, and clinical improvement defined as ≥ 80% improvement in SDAI or DAS28 [CRP]).
Results: Pts with BL erosions were more likely to have new erosions at wk 52 vs pts without BL erosions in both the ABA + MTX (41.5 vs 20.3%) and PBO + MTX (58.3 vs 28.6%) arms; rates were numerically lower with ABA + MTX vs PBO + MTX (Table 1A). Rates of new erosions were also higher in those with ES ≥ 3 (vs < 3) at BL, and were numerically lower in each subgroup with ABA vs PBO (Table 1B). In the BL erosion present (Table 1A) or high BL erosion groups (Table 1B), the proportions of pts with ES increasing by ≥ 3 in the PBO + MTX arm were at least twice as high as in the ABA + MTX arm. Overall, progression of structural damage was less pronounced in pts in the ABA + MTX vs PBO + MTX arm (ES increase 1–2: 4.4 vs 9.7%, respectively; ES increase ≥ 3: 6.5 vs 18.9%, respectively; Table 1A, 1B).
Changes in ES from BL to wk 52 and clinical outcomes at wk 52 are shown for pts by BL ES presence/absence (Table 2A) and high/low status (Table 2B). At wk 52, SDAI remission rates were higher in low vs high BL ES groups in both arms (Table 2B). Overall, clinical response rates were higher in the ABA + MTX vs PBO + MTX arm (SDAI remission: 36.8 vs 22.0%, respectively; DAS28 (CRP) < 2.6: 58.8 vs 42.7%, respectively; clinical improvement: 61.4 vs 43.2%, respectively; Table 2A, 2B).
Conclusion: In ACPA+ pts with early RA, abatacept + MTX was more effective than MTX alone in slowing the progression of structural damage and improving clinical response over 52 wks, with greater progression of structural damage associated with presence and severity of BL erosions.
The greater progression of structural damage in pts with high BL erosions receiving MTX alone vs abatacept + MTX reinforces the need to treat such pts earlier with biologic DMARDs such as abatacept to better preserve their joints.
1Emery P, et al. Arthritis Rheum 2018;70.abs563.
2Aletaha D, et al. Arthritis Rheum 2010;62:2569–2581.
Medical writing: Joanna Wright (Caudex), funded by Bristol Myers Squibb.
To cite this abstract in AMA style:
Pachai C, Connolly S, Tanaka Y, Bykerk V, Huizinga T, Citera G, Bingham III C, Du S, Hoexter G, Fleischmann R, Banerjee S, Emery P. Reduction in Structural Damage Progression and Improvement in Clinical Response with Abatacept Treatment in Patients with ACPA+, Early RA: Results from a Post Hoc Analysis of the AVERT-2 Study by Baseline Erosion Scores [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reduction-in-structural-damage-progression-and-improvement-in-clinical-response-with-abatacept-treatment-in-patients-with-acpa-early-ra-results-from-a-post-hoc-analysis-of-the-avert-2-study-by-base/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-structural-damage-progression-and-improvement-in-clinical-response-with-abatacept-treatment-in-patients-with-acpa-early-ra-results-from-a-post-hoc-analysis-of-the-avert-2-study-by-base/