Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Diagnosis, Manifestations, and Outcomes III: RA ILD
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Clinically significant interstitial lung disease (ILD) occurs in roughly 10% of patients with rheumatoid arthritis (RA). There are limited data on the pathogenesis of RA-ILD; risk factors include smoking, rheumatoid factor or anti-cyclic citrullinated peptide autoantibodies, and genetic variants including MUC5B, HLA-B54, HLA-B40, and HLA-DQ1B*060. Current data are lacking regarding the risk of biologic and targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) use on the development, and/or exacerbation, of RA-ILD. The objective of this nationwide, retrospective study was to estimate the incidence of ILD in RA patients treated with different b/tsDMARDs and to compare the risk of developing ILD between patients treated with different b/tsDMARDs and adalimumab.
Methods: This retrospective cohort study used the Optum Clinformatics® Data Mart (2003-2019). Adult patients with RA, ≥1 year of continuous enrollment, treatment with a b/tsDMARD of interest, and without preexisting ILD were included. Crude incidence rates (IRs) for the development of ILD were calculated. The risk of ILD across different b/tsDMARDs was compared using Cox-regression models. A sensitivity analysis using a prevalent new-user cohort design compared patients treated with tofacitinib with patients treated with adalimumab at a similar timepoint in their disease course.
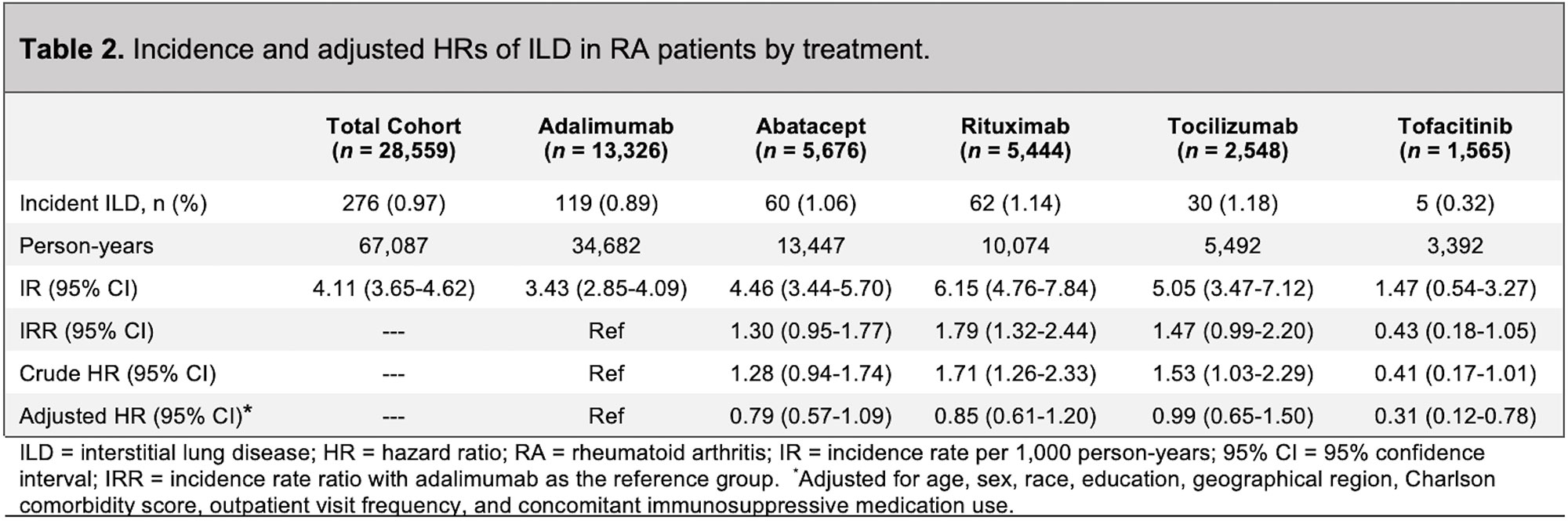
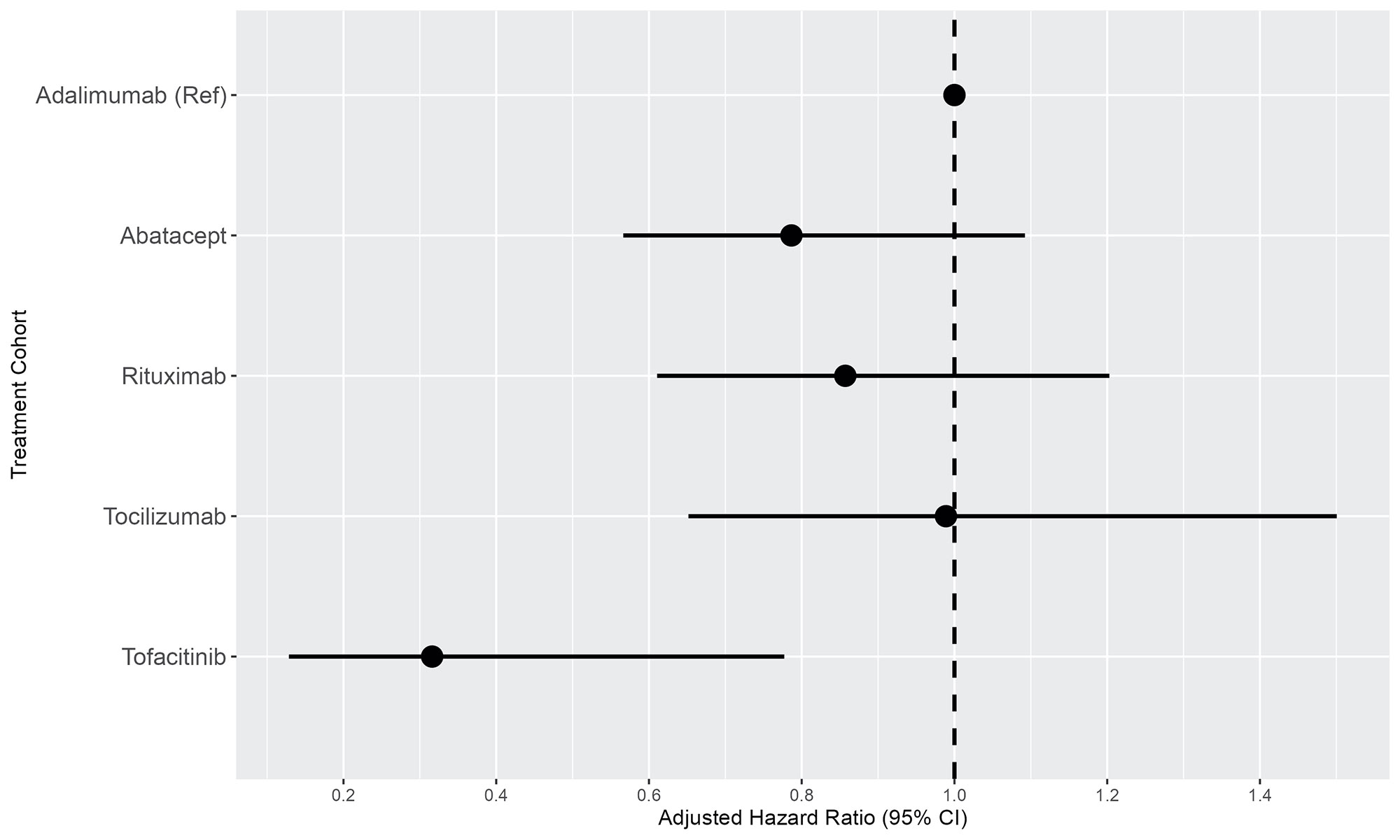
Results: A total of 28,559 RA patients (mean age 56 years; 78% female) were treated with adalimumab (n=13,326), abatacept (n=5,676), rituximab (n=5,444), tocilizumab (n=2,548), or tofacitinib (n=1,565) (Table 1). The crude IRs per 1,000 person-years of ILD were 3.43 (95% CI 2.85-4.09) for adalimumab, 4.46 (95% CI 3.44-5.70) for abatacept, 6.15 (95% CI 4.76-7.84) for rituximab, 5.05 (95% CI 3.47-7.12) for tocilizumab, and 1.47 (95% CI 0.54-3.27) for tofacitinib (Table 2). After multiple adjustments, compared with patients treated with adalimumab, patients treated with tofacitinib had a lower risk of ILD (adjusted hazard ratio [aHR] 0.31, 95% CI 0.12-0.78, p=0.009) (Table 2 and Figure 1). Utilizing the prevalent new-user cohort design, after 3:1 propensity score matching, cohorts of 4,677 patients treated with adalimumab and 1,559 patients treated with tofacitinib were constructed (mean age 58 years, 83% female, 11 outpatient visits per year, roughly 38% methotrexate use for both groups). The IR per 1,000 person-years of ILD for RA patients treated with tofacitinib was 1.48 (95% CI 0.54-3.28), compared with 4.30 (95% CI 3.15-5.74) for adalimumab. In the adjusted model, there was a 67% reduction in risk for the development of ILD in patients treated with tofacitinib compared with adalimumab (aHR 0.33, 95% CI 0.13-0.83, p=0.019).
Conclusion: In this large, retrospective nationwide study, we demonstrated a significant reduction in the risk of developing ILD in RA patients treated with tofacitinib. There are currently no guidelines for the treatment of patients with RA at risk for developing ILD or with existing ILD. These results suggest that treatment with tofacitinib, and perhaps other Janus kinase inhibitors, may provide benefit in reducing the risk of developing ILD and could be considered for treating patients with RA and preexisting ILD as well.
To cite this abstract in AMA style:
Baker M, Liu Y, Lu R, Lin J, Melehani J, Robinson W. Reduction in Rheumatoid Arthritis-Associated Interstitial Lung Disease Risk in Patients Treated with Tofacitinib [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reduction-in-rheumatoid-arthritis-associated-interstitial-lung-disease-risk-in-patients-treated-with-tofacitinib/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reduction-in-rheumatoid-arthritis-associated-interstitial-lung-disease-risk-in-patients-treated-with-tofacitinib/