Session Information
Date: Saturday, November 6, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Impact (0225–0240)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
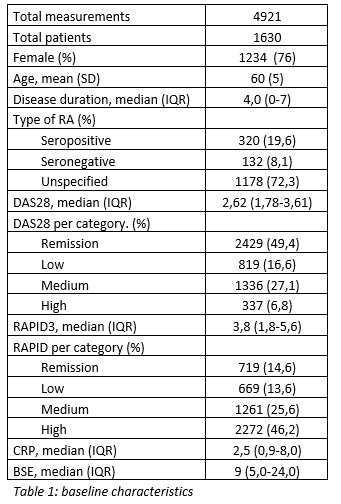
Background/Purpose: The long-term and frequent evaluation of disease activity in patients with rheumatoid arthritis (RA) leads to a large burden of planned consultations at outpatient clinics. It might be possible to reduce that burden by prescreening for patients in remission or low disease activity with the electronic Routine Assessment of Patient Index Data 3 (RAPID3). For this purpose, accurate classification of patients in the low or remission category with the RAPID3 is a necessity. The objective is to evaluate the test characteristics and agreement in the low disease activity categories between the Disease Activity Score 28 (DAS28) and the RAPID3 in patients with RA.
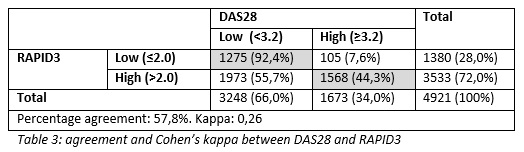
Methods: We performed a retrospective database study with available clinical data collected as part of usual care from the electronic medical record at Reade Amsterdam. The dataset comprised each completed RAPID3 between June 2014 and March 2021, followed by a DAS28 within 2 weeks in patients with RA. The disease activity categories for both the RAPID3 and DAS28 were dichotomized in low (remission and low disease activity) and high (moderate and high disease activity). Cutoff values were 2.0 for RAPID3 and 3.2 for DAS28. Test characteristics and agreement (Cohen’s kappa) were calculated.
Results: A total of 4921 combined RAPID3 and DAS28 measurements were done at Reade in 1630 unique RA patients. The mean age was 60 years and 76% were female with a median disease duration of 4 years. The agreement was considered fair (ƙ=0,260). In total, 1630 (28%) of the RAPID3 measurements were classified as low. The sensitivity to detect low disease activity was 0.39, specificity was 0.94 and the positive predicted value was 0.92.
Conclusion: We showed that when the RAPID3 classifies a patient into the remission or low disease activity state, it is for 92% accurate. Of all consultations, 28% could possibly be postponed following screening with RAPID3. We propose a system where the RAPID3 is one of a few conditions that need to be met before consultations are postponed. Other conditions could include patient-, and physician approval and within-range lab results. Future research needs to focus on the feasibility of this system and finding the proper conditions for it to safely and effectively reduce the number of consultations.
To cite this abstract in AMA style:
Wiegel J, Seppen B, ter wee M, Boers M, Nurmohamed M, Bos w. Reducing the Number of Outpatient Clinic Visits by Using the Routine Assessment of Patient Index Data 3 as a Screening Tool [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/reducing-the-number-of-outpatient-clinic-visits-by-using-the-routine-assessment-of-patient-index-data-3-as-a-screening-tool/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reducing-the-number-of-outpatient-clinic-visits-by-using-the-routine-assessment-of-patient-index-data-3-as-a-screening-tool/