Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: Pegloticase, a recombinant, pegylated uricase, is used for treatment of gout in patients who fail oral urate lowering therapy (ULT). Despite successful reduction of urate levels and flares, its use is limited due to immunogenicity leading to infusion reactions.1 Co-administration of an immunomodulatory agent may mitigate this loss of efficacy and concern for drug-related toxicity.
Methods: Patients from 5 rheumatology practices were recruited over 18 months, and randomized (3:1) by site to either to mycophenolate mofetil (MMF) 1 gram twice a day or placebo (PBO) with a run-in of 2 weeks (wks) prior to intravenous pegloticase at 8 mg every 2 wks (12 infusions). MMF or PBO was given for the first 12 wks. Inclusion criteria were: a) patients ≥ 18 years of age who met 2015 ACR/EULAR gout classification criteria and b) chronic refractory gout defined as symptoms inadequately controlled with ULT or contraindication to ULT. Endpoints included: a) proportion of subjects achieving and maintaining a serum urate (sUA) ≤ 6 mg/dL at 12 wks (primary), b) assess 6-month durability of immune modulation after discontinuation of MMF at 12 wks (secondary), and c) adverse events (AE). Analyses were conducted using SAS (Cary, NC) Version 9.4 with Fisher’s exact test for binary outcomes and Wilcoxon two-sample test for continuous outcomes. Kaplan-Meier estimates and log-rank test were performed to compare survival curves between groups. Hypothesis tests were two-tailed and p-value (p) < 0.05 indicated statistical significance.
Results: Of 42 subjects screened, 35 were randomized, and 32 who received at least one dose of pegloticase were included in modified intention to treat analyses (Table 1- demographics). 19 of 22 (86%) in the MMF arm achieved the primary outcome at 12 wks of sUA ≤ 6mg/dL, compared to 4 of 10 (40%) in placebo, p-value 0.01. At wk 24, sUA response was sustained in 68% of MMF arm vs. 30% in placebo. Estimated rates of AEs per month was similar between groups -MMF (0.3) and placebo (0.4). The MMF arm had higher AEs compared to placebo: musculoskeletal (36% vs. 10%), respiratory (18% vs. 0%), and infections (9% vs. 0%). The placebo arm had a greater percentage of infusion reactions (30% vs. 0%). Figure 1 demonstrates that the percentage of subjects maintaining a sUA < 6 mg/dL at 12 wks was significantly higher (p=0.02) in the MMF arm, and a significant difference (p=0.03) at 24 wks indicating sustained benefit from MMF.
Conclusion: Short-term concomitant use of MMF with pegloticase was generally well tolerated in our proof of concept study. It was associated with a statistically significant and clinically meaningful impact on the proportion of subjects achieving and maintaining a sUA ≤6 mg/dL at 24 weeks. To our knowledge, this is the first randomized controlled trial to demonstrate prolonged efficacy of pegloticase with co-administrations of an immunomodulatory agent.
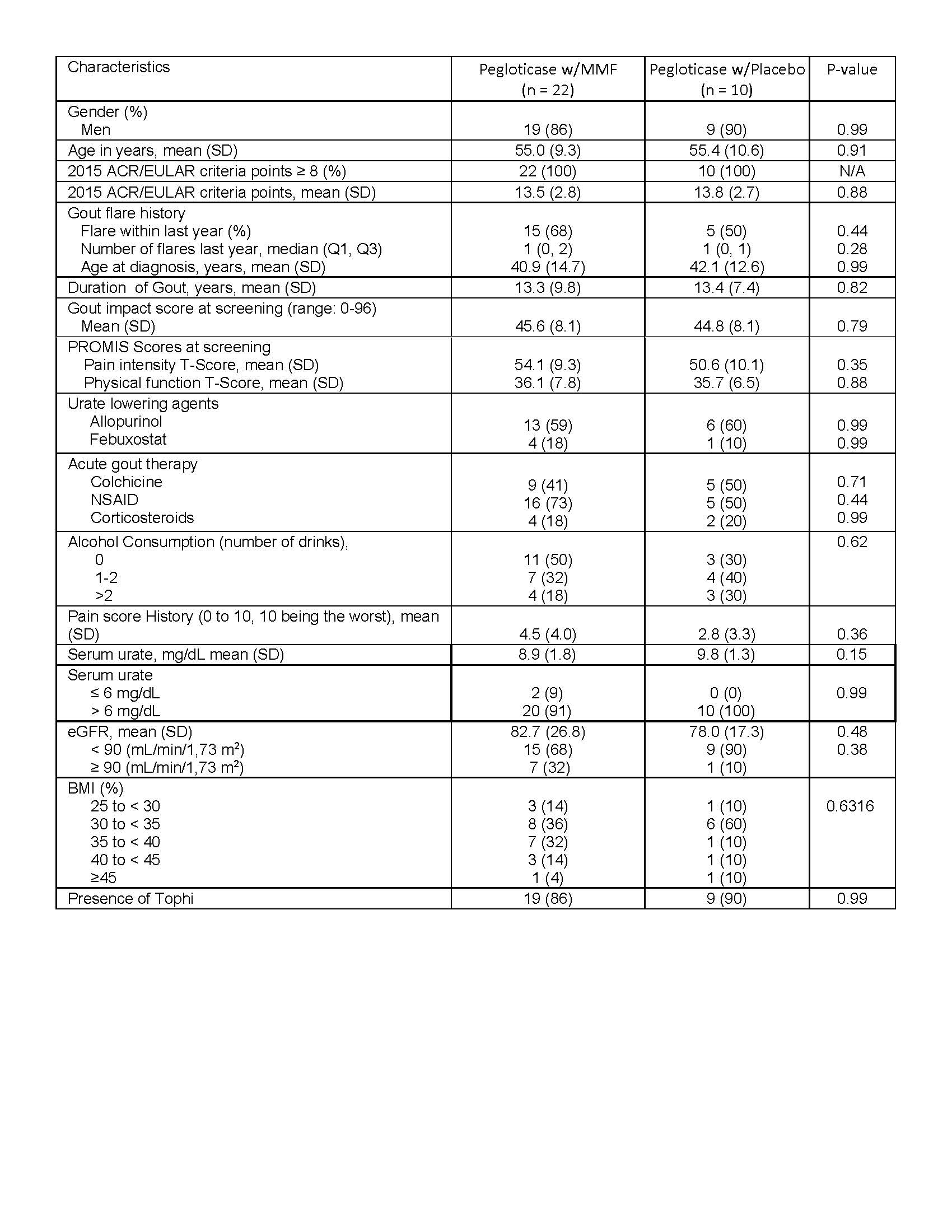 Table 1. Patient demographics for treatment arms of pegloticase with MMF and pegloticase with placebo
Table 1. Patient demographics for treatment arms of pegloticase with MMF and pegloticase with placebo
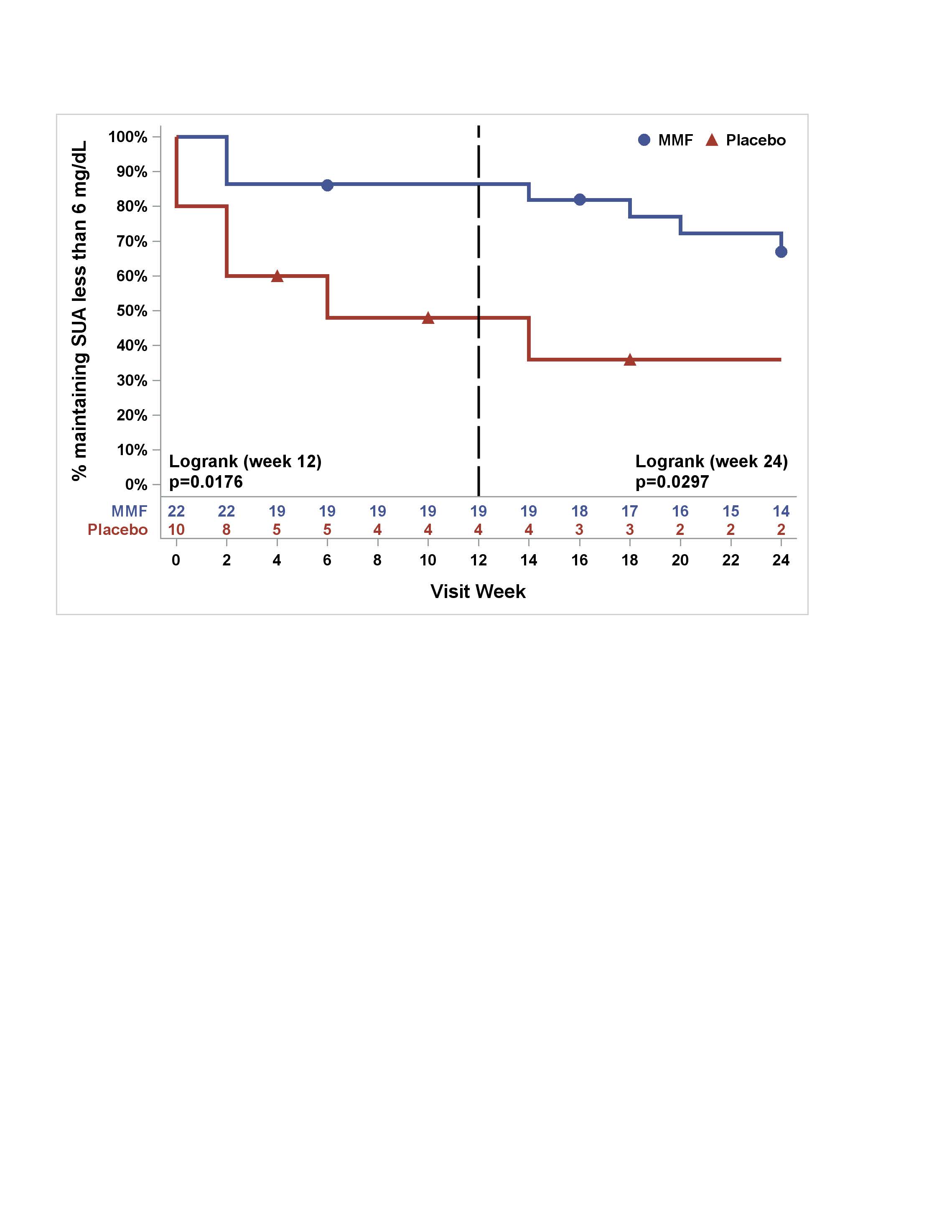 Figure 1. Percentage of subjects maintaining SUA less than 6 mg/dL in MMF/pegloticase vs PBO/pegloticase arms over 24 Weeks (Kaplan‐Meier Estimates)
Figure 1. Percentage of subjects maintaining SUA less than 6 mg/dL in MMF/pegloticase vs PBO/pegloticase arms over 24 Weeks (Kaplan‐Meier Estimates)
To cite this abstract in AMA style:
Khanna P, Khanna D, Cutter G, Foster J, Melnick J, Jaafar S, Biggers S, Rahman A, Kuo H, Feese M, Saag K. Reducing Immunogenicity of Pegloticase (RECIPE) with Concomitant Use of Mycophenolate Mofetil in Patients with Refractory Gout—a Phase II Double Blind Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/reducing-immunogenicity-of-pegloticase-recipe-with-concomitant-use-of-mycophenolate-mofetil-in-patients-with-refractory-gout-a-phase-ii-double-blind-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reducing-immunogenicity-of-pegloticase-recipe-with-concomitant-use-of-mycophenolate-mofetil-in-patients-with-refractory-gout-a-phase-ii-double-blind-randomized-controlled-trial/
