Session Information
Date: Monday, November 6, 2017
Title: Osteoarthritis – Clinical Aspects Poster I: Clinical Trials and Interventions
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
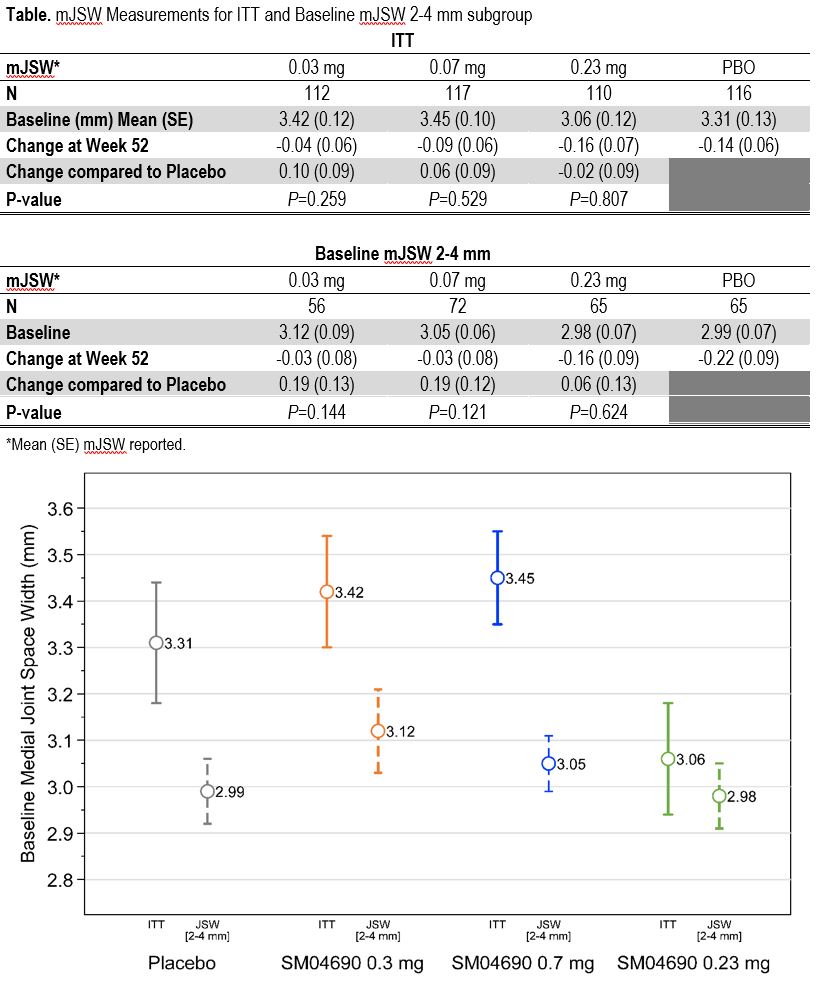
Background/Purpose: Kellgren-Lawrence [KL] radiographic grading is used to classify knee osteoarthritis (OA), but may not accurately reflect disease progression. Classifying subjects by baseline medial joint space width (mJSW) may be a more specific measure. This hypothesis was assessed in a post-hoc analysis of data from a phase 2, multicenter, 52-week, randomized controlled trial of SM04690, a small molecule Wnt pathway inhibitor in development as a potential disease modifying drug in knee OA.
Methods: In the trial, subjects with KL grade 2-3 knee OA were randomized to a single, 2 mL, intra-articular injection of 0.03 mg, 0.07 mg, 0.23 mg SM04690 or placebo (PBO) into their target (most painful) knee at Day 0. WOMAC Pain and Function were assessed at 0, 4, 13, 26, 39 and 52 weeks, with fixed location radiographic assessment of mJSW at Weeks 0, 26 and 52. Exploratory analysis of clinical outcomes in the intention to treat (ITT) population was conducted by analysis of covariance adjusted for baseline mJSW with multiple imputation. This post-hoc analysis examined a group with baseline mJSW of 2-4 mm in comparison to the full ITT population.
Results: 455 subjects (mean age 60.3 [±8.7] years, BMI 29.9 [±4.6] kg/m2, 268 [58.9%] female, 293 [64.4%] KL Grade 3) were enrolled. Contralateral knee KL grade was equal or worse than target knee in 91% of ITT population. 258 subjects had baseline mJSWs of 2-4 mm. At week 52, in the placebo group, imputed mean mJSW change from baseline was -0.14 [SE 0.06] mm. In the ITT population, compared to placebo, imputed mean mJSW changes from baseline were positive for 0.03 mg and 0.07 mg SM04690 doses (Table, figure). In the post-hoc analysis of the smaller 2-4mm mJSW subgroup, heterogeneity was similar to ITT for all doses compared to PBO and for the 0.03 mg and 0.07 mg doses changes beyond measurement error (>0.13 mm)1 were observed. In addition, improvement in WOMAC Function compared to placebo was seen in the 0.07 mg SM04690 group at Week 52 within the mJSW subgroup (change compared to placebo -13.6, 95% CI (-25.5, -1.7), P=0.025).
Conclusion: Stricter inclusion criteria for mJSW provided a less heterogenous baseline group, reducing sample size by 42% without increasing standard error. When applied to this dataset, meaningful radiographic changes were demonstrated with 0.03 mg and 0.07 mg SM04690 groups compared to placebo. Future trials of structure modification in knee OA should consider specific mJSW inclusion criteria.
1Dupuis et al. OAC 2003
To cite this abstract in AMA style:
Conaghan PG, DiFrancesco A, Swearingen CJ, Kennedy S, Simsek I, Tambiah J, Yazici Y. Reducing Heterogeneity in OA Clinical Trials: Data from a Phase 2 Study of SM04690, a Novel, Intra-Articular, Wnt Pathway Inhibitor in Knee Osteoarthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/reducing-heterogeneity-in-oa-clinical-trials-data-from-a-phase-2-study-of-sm04690-a-novel-intra-articular-wnt-pathway-inhibitor-in-knee-osteoarthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reducing-heterogeneity-in-oa-clinical-trials-data-from-a-phase-2-study-of-sm04690-a-novel-intra-articular-wnt-pathway-inhibitor-in-knee-osteoarthritis/