Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Treatment III: Comorbidities and Consequences
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Recent large-scale randomized trials demonstrate that immunomodulators reduce the risk of cardiovascular (CV) events among the general population with atherosclerosis. However, it is uncertain whether these effects apply to rheumatoid arthritis (RA). We conducted a single-blind randomized active comparator trial to determine whether adding a TNF inhibitor (TNFi) or sulfasalazine and hydroxychloroquine (triple therapy) to methotrexate (MTX) would result in more significant reduction of arterial inflammation.
Methods: Patients with active RA despite MTX were randomized to addition of a TNFi to MTX or triple therapy for 24 weeks. Baseline and follow-up 18F-FDG PET/CT scans were assessed for changes in arterial inflammation, an index of CV risk, measured as an arterial target-to-background ratio in the most disease segment (TBR MDS) in the carotid arteries and aorta. We also examined differences between baseline and final follow-up values for CV risk factors between the two treatment groups.
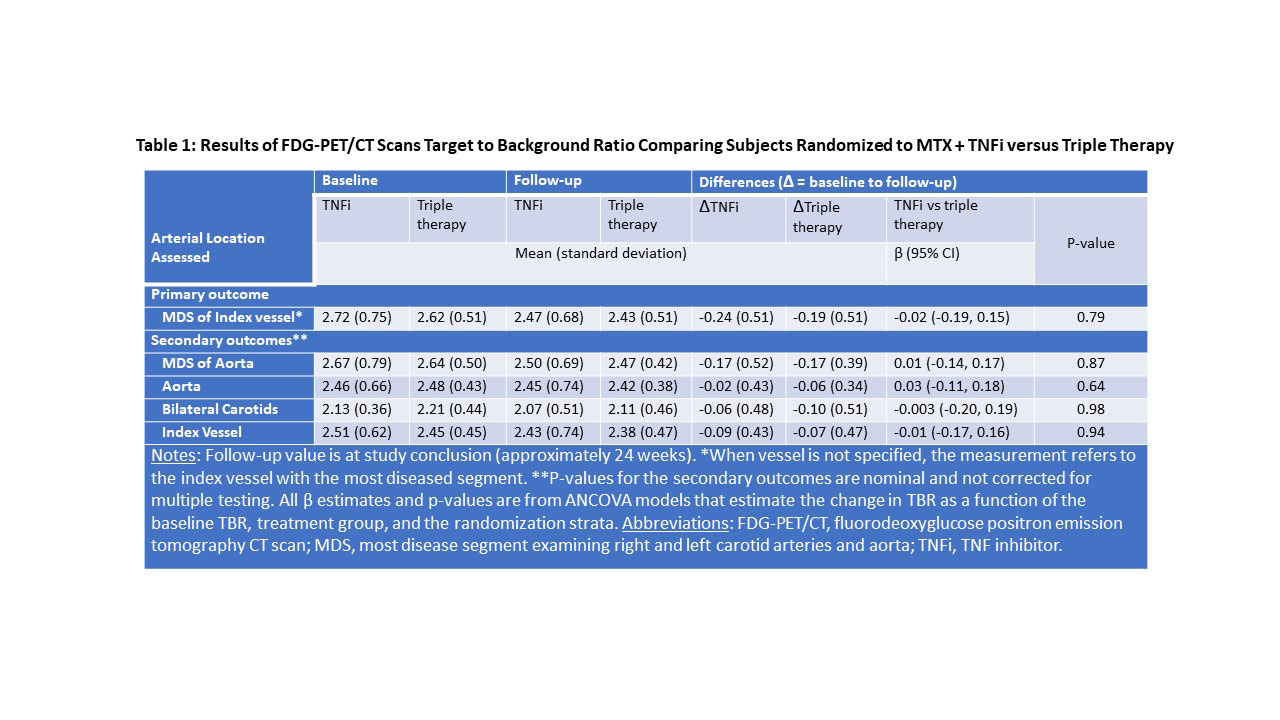
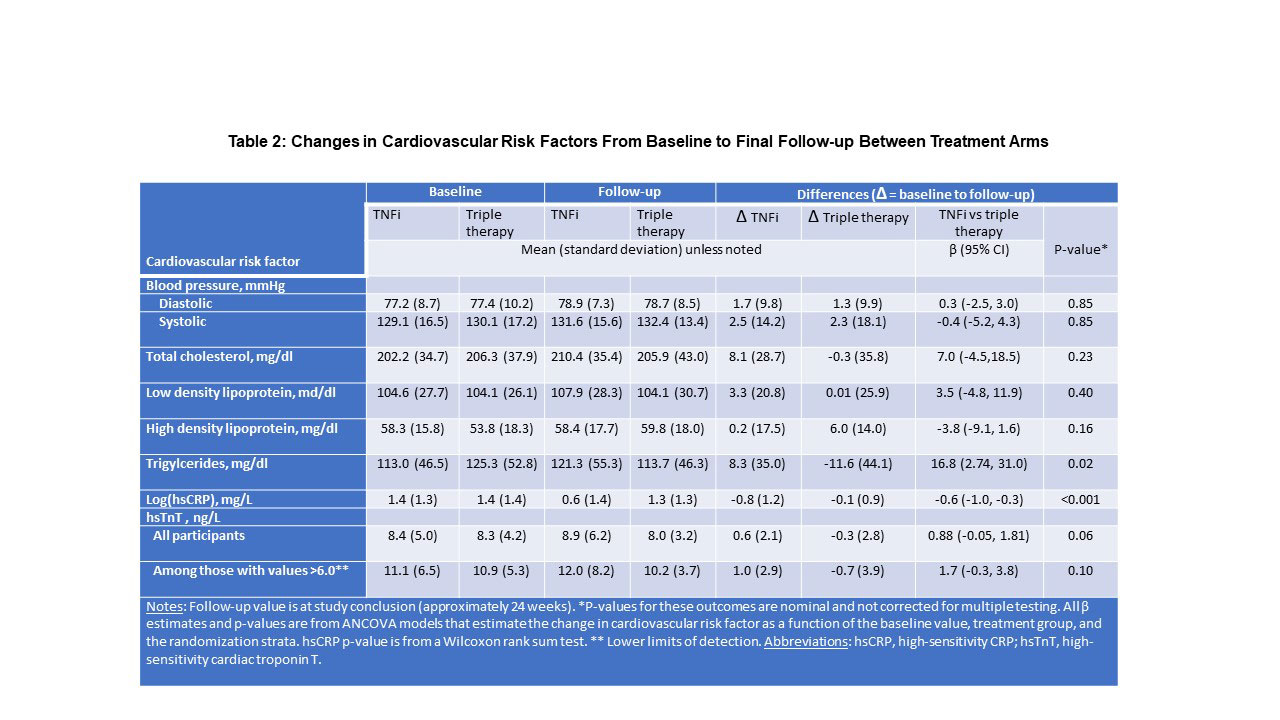
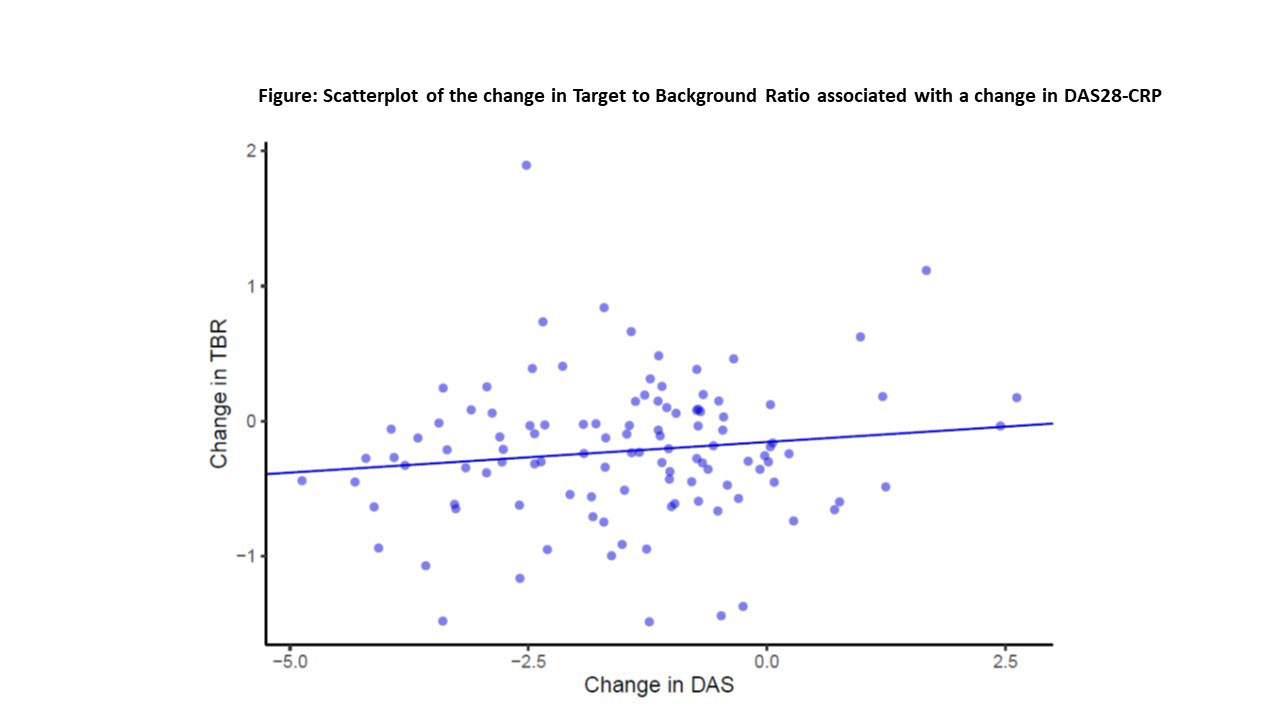
Results: Of 159 participants randomized, 115 (72%) completed the protocol (58 received TNFi and 57 triple therapy) with scans that could be evaluated for the primary outcome. The two treatment groups were well balanced with a mean age of 58 years, 71% female, 57% seropositive. Median baseline DAS28-CRP was 4.8 (IQR 4.0,5.6), indicating moderate disease activity, despite a median weekly dosage of MTX 20mg. Baseline TBR MDS was similar and elevated across both treatment groups. Significant reductions in TBR MDS were observed across both groups: ΔTNFi -0.24 (SD = 0.51; p = 0.001) and Δtriple therapy -0.19 (SD = 0.51; p = 0.001). Yet, we observed no significant difference between treatment groups in the TBR MDS (difference in Δs -0.02, 95% CI -0.19, 0.15, p = 0.79) or in any pre-specified secondary outcomes (see Table 1). DAS28-CRP was significantly reduced across both treatment groups, and there was no correlation between the decline in DAS28-CRP and change in TBR MDS (β = 0.04, 95% CI -0.03, 0.10) (see Figure). With respect to CV risk factors, triglycerides decreased in the triple therapy group (-11.6 mg/dl) more than with TNFi (+8.3 mg/dl; p = 0.02) and log(hsCRP) decreased more in the TNFi group than triple therapy (p< 0.001). Changes in other CV risk factors did not differ by treatment group (see Table 2).
Conclusion: Among patients with RA with at least moderate disease activity despite MTX, addition of either a TNFi or triple therapy resulted in significant improvement in arterial inflammation. However, the addition of a TNFi did not reduce arterial inflammation more than triple therapy. Further, there was no significant correlation between changes in disease activity and arterial inflammation.
To cite this abstract in AMA style:
Solomon D, Giles J, Liao K, Ridker P, Rist P, Glynn R, Broderick R, Lu F, Murray M, Vanni K, Santacroce L, Abohashem S, Robson P, Fayad Z, Mani V, Tawakol A, Bathon J. Reducing Cardiovascular Risk with Immunomodulators: A Single Blind Randomized Active Comparator Trial Among Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reducing-cardiovascular-risk-with-immunomodulators-a-single-blind-randomized-active-comparator-trial-among-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reducing-cardiovascular-risk-with-immunomodulators-a-single-blind-randomized-active-comparator-trial-among-patients-with-rheumatoid-arthritis/