Session Information
Date: Monday, November 14, 2022
Title: Abstracts: RA – Treatment III: Comorbidities and Consequences
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Janus kinase (JAK) inhibitors are known to increase the risk of herpes zoster (HZ) reactivation; however, studies on the risk of HZ recurrence when JAK inhibitors are continued after an episode of HZ reactivation are scarce. Therefore, we conducted a nationwide population-based study on the safety of continued use of JAK inhibitors in patients with immune-mediated inflammatory diseases (IMID) who develop HZ reactivation.
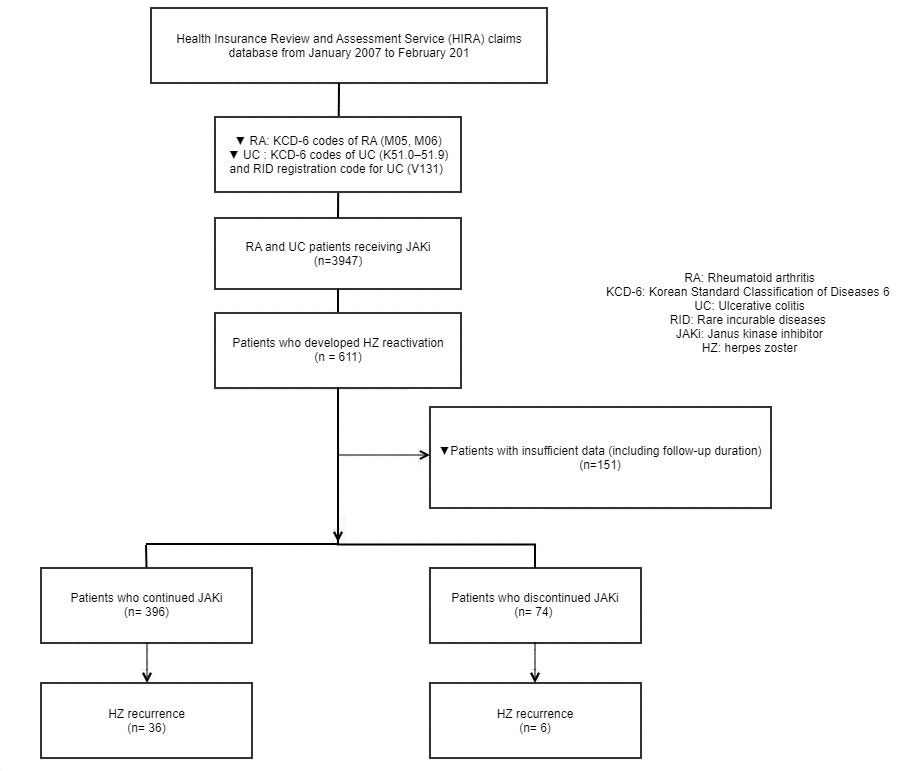
Methods: Medical data from the Korean Health Insurance Review and Assessment Service (HIRA) of patients using JAK inhibitors for the treatment of rheumatoid arthritis (RA) and ulcerative colitis (UC) between 2007 and 2021 were analyzed.
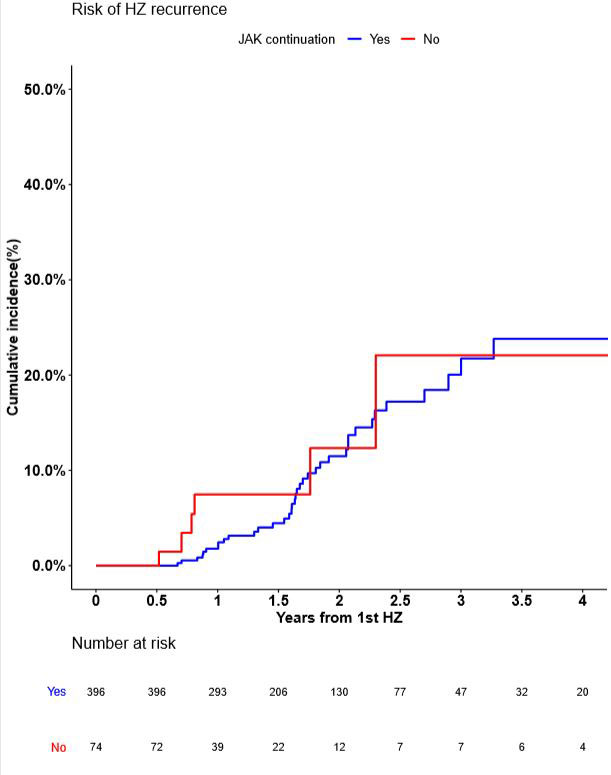
Results: A total of 3947 patients receiving JAK inhibitors were included, of whom 2827 (71.6%) and 1120 (28.4%) patients received tofacitinib and baricitinib, respectively. After a median of 0.95 years of exposure (IQR, 0.93-2.58), 611 patients (15.4%) developed HZ reactivation, with an incidence rate of 8.38 per 100 person-years. One hundred and fifty one patients were excluded from the study because of lack of data including follow-up duration (less than 180 days after HZ reactivation). Patients who continued use of JAK inhibitors numbered 386 of 470 (83.9%), while the remaining 74 (16.1%) discontinued JAK inhibitors. During a median follow-up of 1.11 years (IQR, 0.53-1.91), 42 patients experienced a recurrence of HZ (JAK inhibitors continuation, n=36; JAK inhibitors discontinuation, n=6). The rate of HZ recurrence in the JAK inhibitor continuation and discontinuation groups were 5.47 per 100 person-years and 5.90 per 100 person-years, respectively, which were not statistically significant (P=0.57). The two groups had no significant difference in terms of medication use other than TNF inhibitors (P < .0001) including conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) (P=0.555) and glucocorticoids (P=0.057) from initial HZ reactivation to recurrent HZ. Lastly, when we examined risk factors for the development of recurrent HZ following HZ reactivation according to the continuation of JAK inhibitors, continued JAK inhibitors was not associated with a higher risk for HZ reactivation (adjusted HR, 0.75 [CI, 0.31 to 1.79], p=0.52).
Conclusion: Among the patients with IMID who experienced HZ reactivation while using JAK inhibitors, more than four-fifths continued using JAK inhibitors. Regardless of the continuation or discontinuation of JAK inhibitors, the recurrence rate of HZ was not significantly different, and continued JAK inhibitors was not associated with a higher risk for HZ reactivation. These results suggested that JAK inhibitor use can be continued even after HZ reactivation.
To cite this abstract in AMA style:
Kim Y, Kim Y, Kim S, Ahn S, Oh J, Kim Y, Lee C, Yoo B, Park S, Hong S. Recurrence of Herpes Zoster Reactivation After Events of Herpes Zoster in Patients Receiving JAK Inhibitors in South Korea: A Nationwide Population-based Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/recurrence-of-herpes-zoster-reactivation-after-events-of-herpes-zoster-in-patients-receiving-jak-inhibitors-in-south-korea-a-nationwide-population-based-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recurrence-of-herpes-zoster-reactivation-after-events-of-herpes-zoster-in-patients-receiving-jak-inhibitors-in-south-korea-a-nationwide-population-based-study/