Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Compared to the immunocompetent US population ≥50 years, adults with rheumatic diseases are at increased risk for herpes zoster (HZ). To address this risk, ACIP recommends 2 doses of recombinant zoster vaccine (RZV) for HZ prevention in adults ≥19 years who are or will be immunodeficient or immunosuppressed because of disease or therapy. However, RZV uptake among adults with rheumatic diseases and the perceived role of rheumatologists in vaccinating this population are unclear.
Methods: This mixed methods research involved 2 distinct phases. First, a retrospective cross-sectional analysis using IQVIA open-source medical and pharmacy claims (04/20/2021 to 06/30/2023) described RZV uptake among adults ≥19 years old with >1 diagnosis and/or treatment for rheumatoid arthritis (RA; ICD-10: M05.x, M06.x, M08.x), psoriatic arthritis (PsA; ICD-10: L40.5x), ankylosing spondylitis (ICD-10: M081, M450-M459), or systemic lupus erythematosus (SLE; ICD-10: M32.x) as of October 20, 2021 (date of ACIP vote). Patients were then followed from this date through the earlier of HZ vaccination or end of the study period. Outcomes included RZV uptake proportion (i.e., at least 1 dose), series completion (2 doses), and dosing schedule compliance (second dose within 1-6 months of the initial dose). RZV uptake was assessed descriptively, and Kaplan-Meier analyses estimated time to series completion. A generalized estimating equation, controlling for patient demographics, clinical characteristics, and social determinants of health, predicted the odds of RZV uptake by rheumatic disease. A semi-structured interview guide was developed by the study team and used to gauge perceptions and experiences of rheumatologists with vaccinating their immunocompromised patients. Each phone-based interview was recorded, transcribed, and thematically assessed.
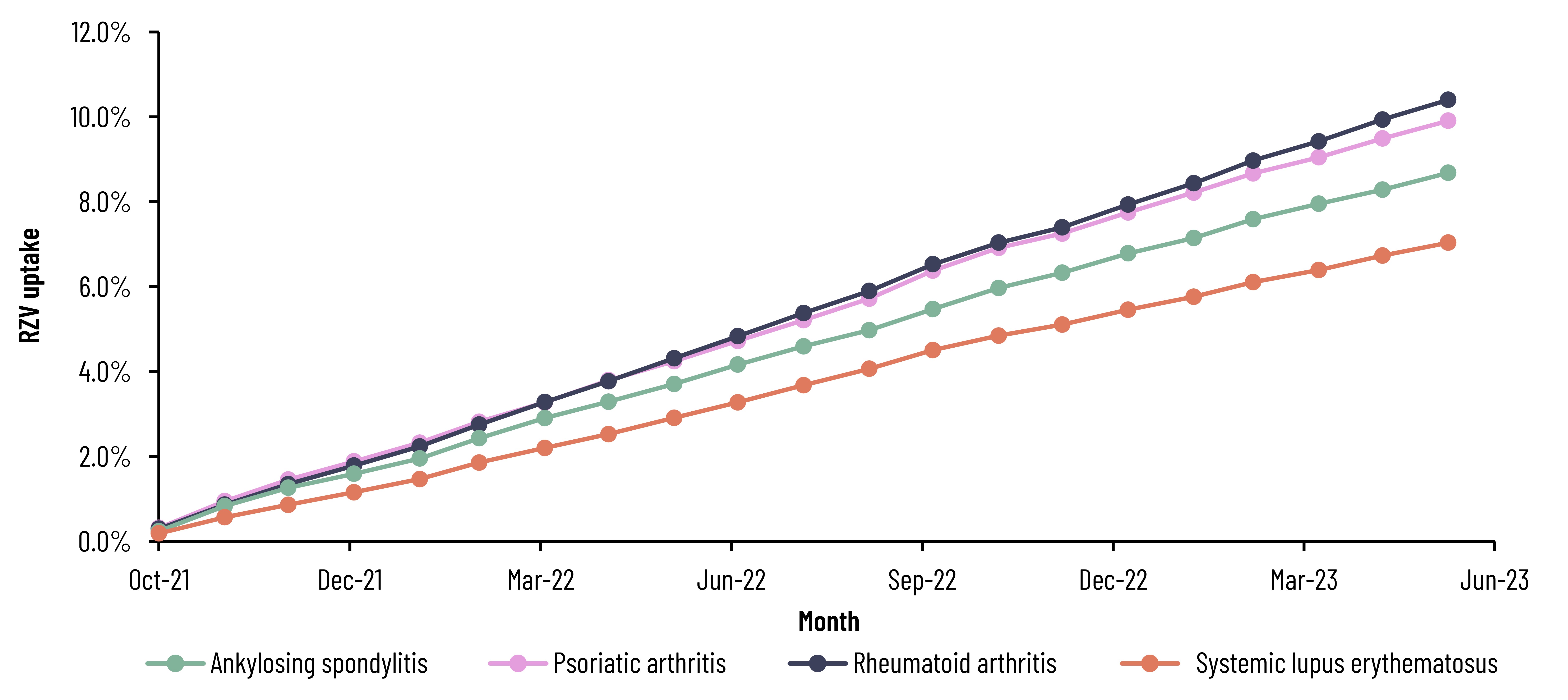
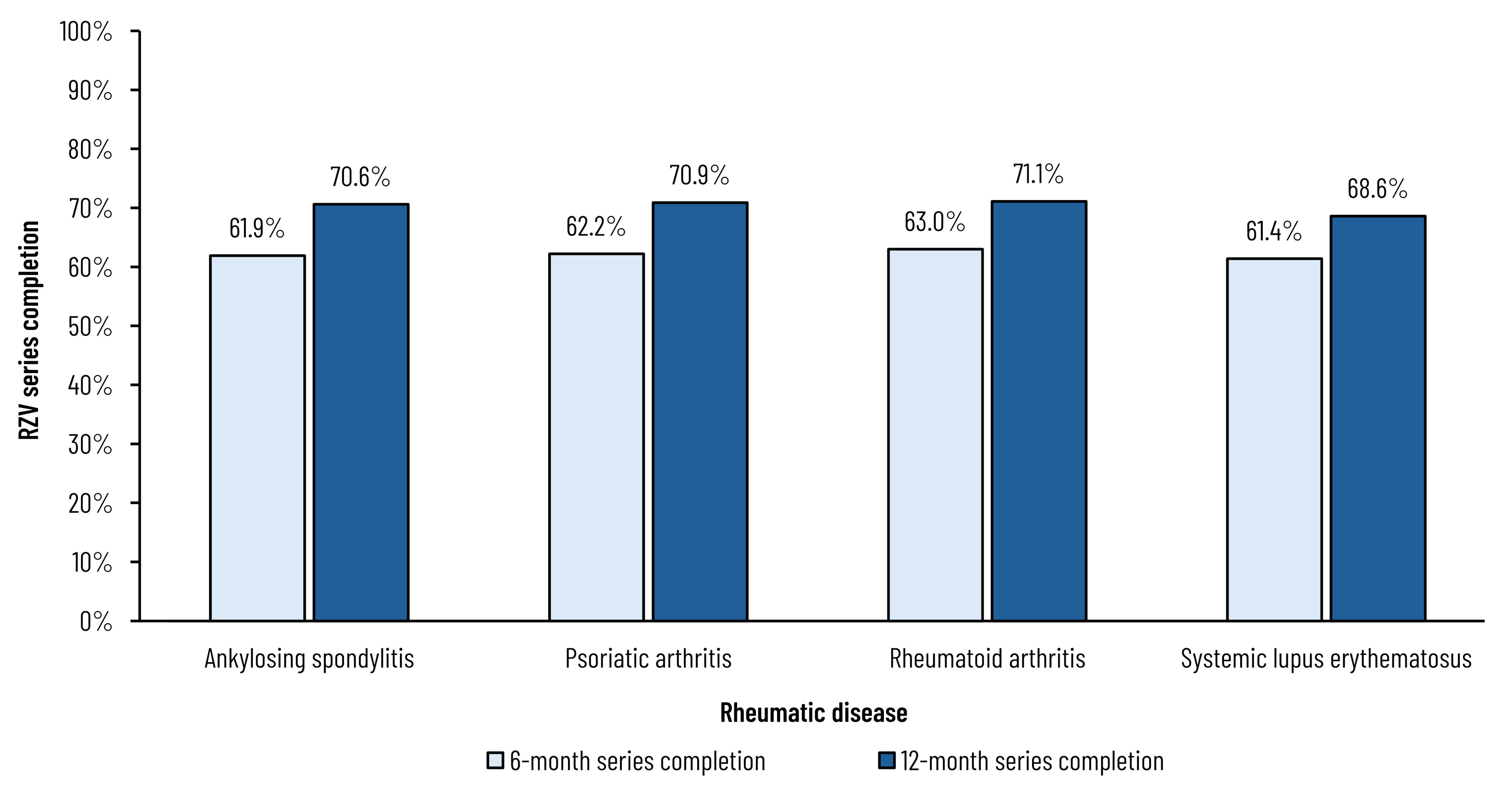
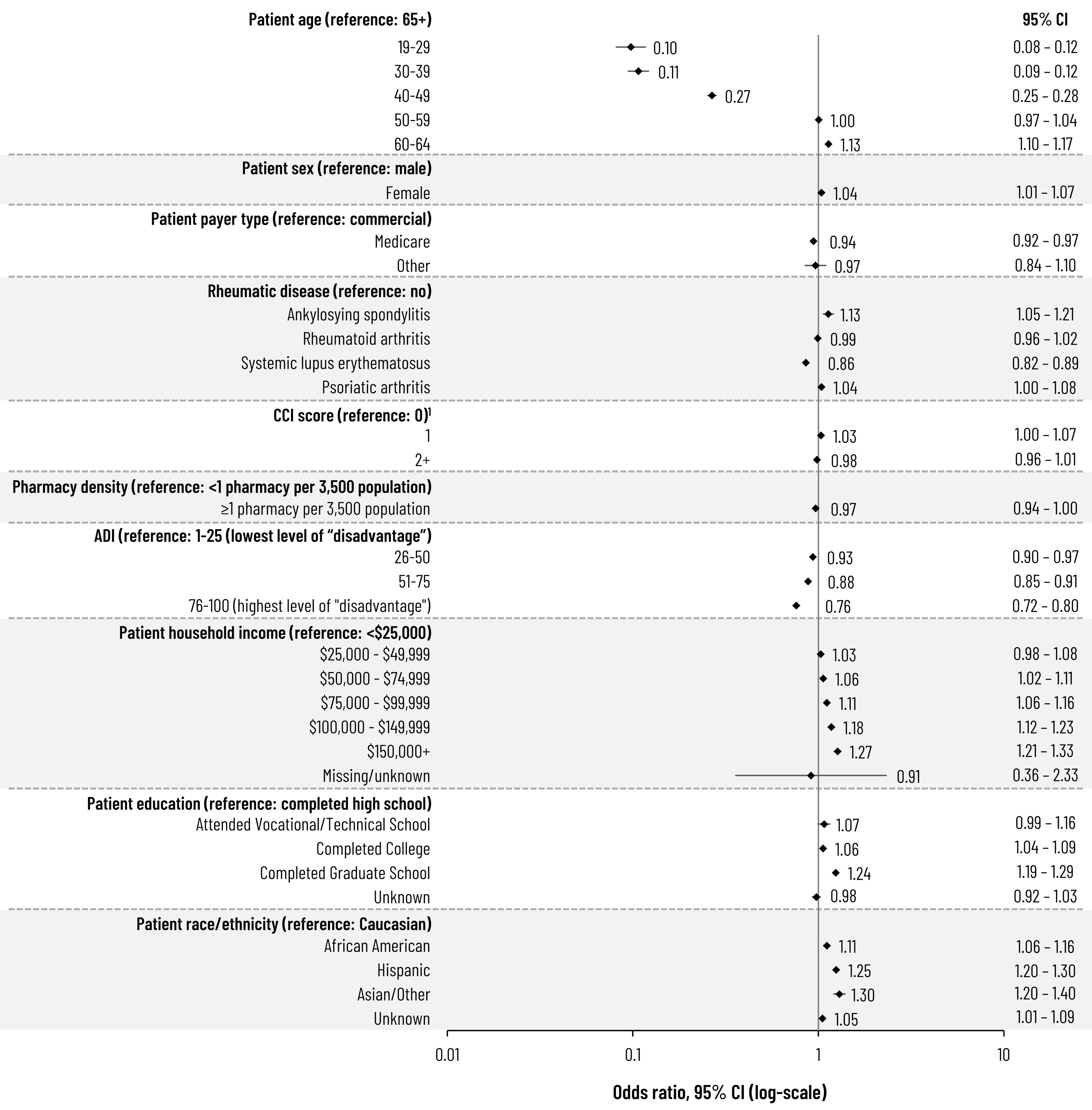
Results: Across the 4 included conditions, 548,426 patients were identified, the majority of which were female (79.1%), had RA (63.1%), and were at least 50 years of age (72.8%). Cumulative RZV uptake was 9.5% and ranged from 7.0% to 10.4% by condition through June 2023 (Figure 1). Among those initiating the RZV series, completion was 62.6% at 6 months, increasing to 70.6% by 12 months post-first dose (Figure 2). Among the included rheumatic conditions, patients with SLE were less likely to receive RZV (odds ratio [OR]: 0.86; 95% confidence interval [CI]: 0.824-0.891) while those with ankylosing spondylitis were more likely (OR: 1.13; 95% CI: 1.055-1.215) (Figure 3). Rheumatologist feedback on vaccination practices (N=10) suggested they understand their patients are at increased risk of HZ but prefer to play a more advisory role, and that inconsistent documentation, poor communication, and competing critical medical needs are barriers to vaccination.
Conclusion: HZ vaccine uptake since the ACIP recommendation remains suboptimal among patients with rheumatic diseases, but once initiated series completion and dosing schedule compliance are likely. Additionally, while rheumatologists may recommend RZV they mostly defer vaccination to other providers.
RZV, recombinant zoster vaccine.
RZV, recombinant zoster vaccine.
Note: Patients vaccinated with at least 1 dose.
ADI, area-deprivation index; CCI, Charlson comorbidity index; CI, confidence interval.
To cite this abstract in AMA style:
Gatwood J, McGuiness C, Yasuda M, Chen C, Gupta V, Stempniewicz N. Recombinant Zoster Vaccine Uptake in US Adults with Rheumatic Disease: A Mixed Methods Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/recombinant-zoster-vaccine-uptake-in-us-adults-with-rheumatic-disease-a-mixed-methods-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recombinant-zoster-vaccine-uptake-in-us-adults-with-rheumatic-disease-a-mixed-methods-analysis/