Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Infections are the leading cause of hospitalization and mortality in patients with ANCA vasculitis (AV). Multiple AV treatment guidelines recommend antimicrobial prophylaxis during treatment with rituximab or cyclophosphamide, with agents that target prevention of Pneumocystis jiroveciipneumonia (PJP), but vary in recommendations for AV patients on other immunosuppressant (IS) medications. Providers may also initiate prophylaxis with other antimicrobials to prevent non-PJP infections. To address a gap in knowledge regarding real-world patterns of prophylaxis in AV, we characterized receipt, type, and duration of antimicrobial prophylaxis in U.S. Medicare beneficiaries initiating IS medication over 2016-18.
Methods: Using Medicare fee-for-service claims and a 90-day washout, we identified a national, retrospective cohort of new IS treatment episodes in 2016-18, including rituximab (RTX), cyclophosphamide (CYC), methotrexate, azathioprine, mycophenolate, leflunomide, mepolizumab, reslizumab, benralizumab, dupilumab, and/or corticosteroids (CS) at >10 mg/day prednisone equivalents. Episodes were restricted to adults with ≥1 diagnosis code for granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), or eosinophilic granulomatosis with polyangiitis (EGPA) in the prior year. IS regimens were classified as having either a strong indication for prophylaxis (regimens containing RTX, CYC, or CS of >20 mg/day in combination with another IS) or weaker indication (all other regimens). Prophylaxis was defined as ≥14 days’ supply of a qualifying antimicrobial overlapping the first 30 days of IS treatment. Antimicrobials included PJP prevention (trimethoprim-sulfamethoxazole, pentamidine, dapsone, atovaquone), other antibacterials (azithromycin, doxycycline, fluoroquinolones), and antifungals (azole or mold-active). We calculated descriptive statistics overall and by IS regimen. We estimated Aalen-Johansen cumulative incidence of prophylaxis discontinuation over one year of follow-up, with death as a competing risk.
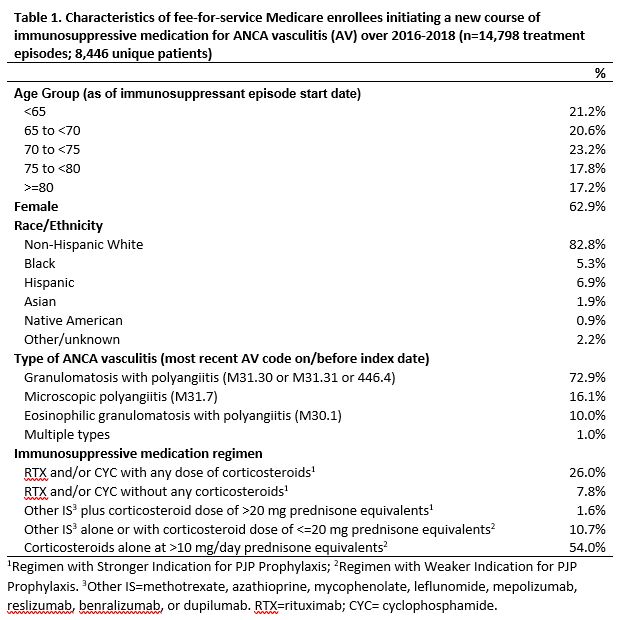
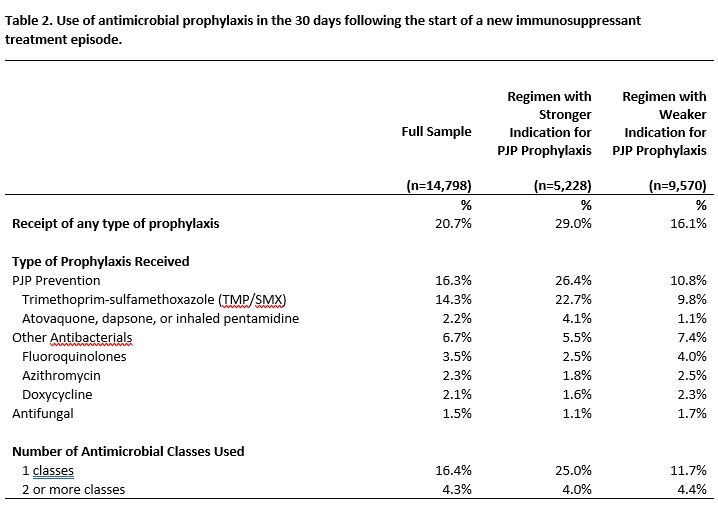
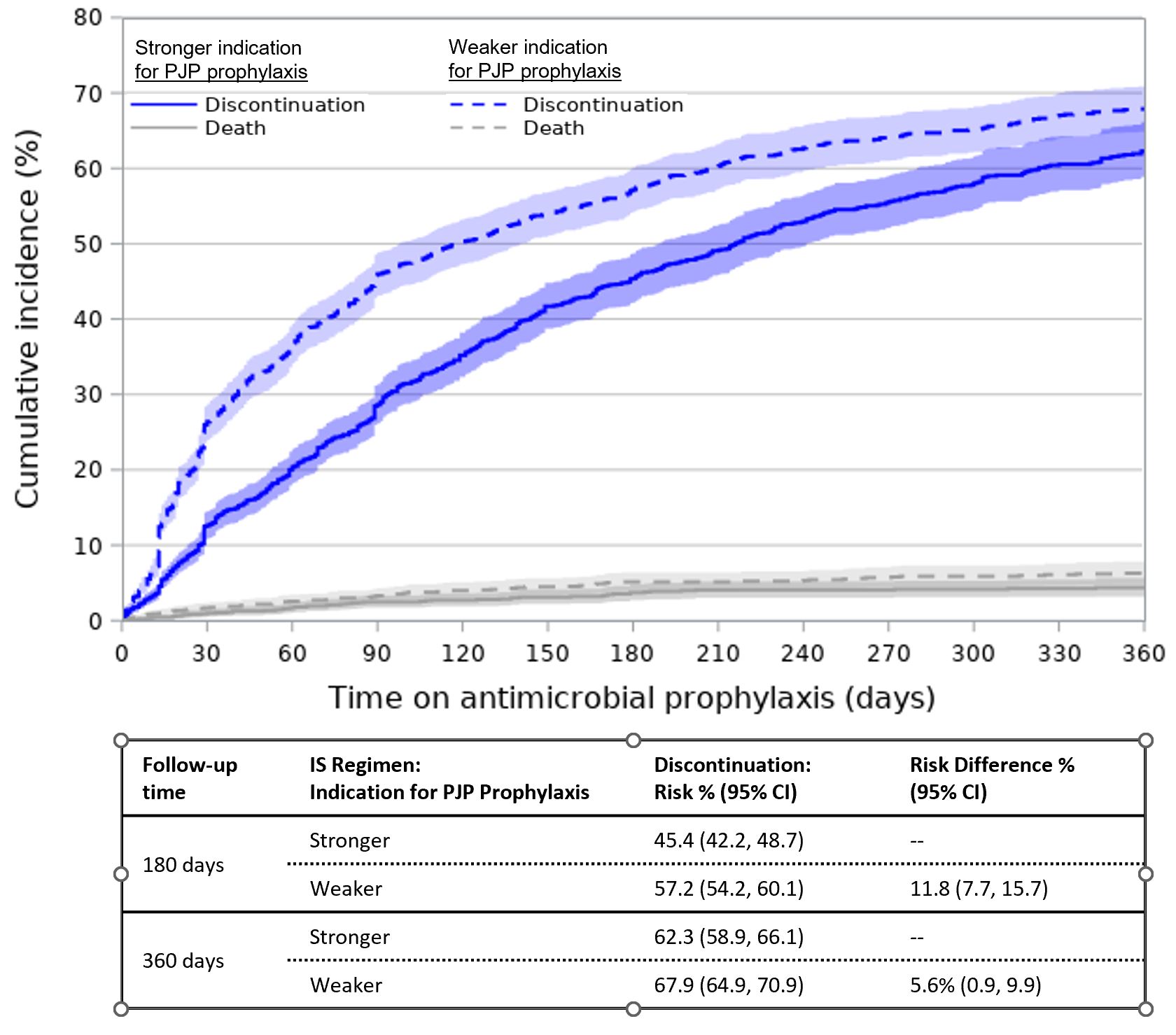
Results: Among 14,798 new AV IS treatment episodes (Table 1), 35% involved regimens with a stronger indication for prophylaxis. Overall, 21% received prophylaxis in the 30 days following IS initiation (Table 2). PJP prophylaxis was most frequent (16%), followed by other antibacterials (7%) and antifungals (2%). IS regimens with a stronger vs. weaker indication for prophylaxis more often involved any prophylaxis (29% vs. 16%) and PJP prophylaxis (26% vs. 11%), but less often involved other antibacterials (5.5% vs. 7.4%). The cumulative incidence of antimicrobial discontinuation was 51.3% (95% CI=49.1, 53.7) at 180 days after IS initiation and 65.1% (95% CI=62.6-67.5) at 360 days, and was higher for regimens with a weaker indication for prophylaxis (360-day RD=5.6%, 95% CI=0.9-9.9; Figure 1).
Conclusion: Despite high infection risk, most AV patients initiating IS did not receive antimicrobials for prevention of PJP or other infections and a majority discontinued within 6 months. This discordance with AV treatment guidelines highlights the need to further investigate barriers and outcomes associated with prophylaxis in this population.
To cite this abstract in AMA style:
Thorpe C, Hickson R, Aspinall S, Derebail V, Zhao X, Thorpe J, Cao B, Ehlert A, Falk R, Hogan S. Receipt of Antimicrobial Prophylaxis in U.S. Medicare Beneficiaries Initiating Immunosuppressive Medications for ANCA Vasculitis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/receipt-of-antimicrobial-prophylaxis-in-u-s-medicare-beneficiaries-initiating-immunosuppressive-medications-for-anca-vasculitis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/receipt-of-antimicrobial-prophylaxis-in-u-s-medicare-beneficiaries-initiating-immunosuppressive-medications-for-anca-vasculitis/