Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The development, exacerbation, or unimprovement of clinical conditions during DMARD treatment may lead patients to abandon treatment before the necessary time has elapsed for drug effectiveness. To better understand the nature of events that lead to early discontinuation, we reviewed b/tsDMARD episodes discontinued by patients with rheumatoid arthritis (RA) while in care of the American Rheumatology Network (ARN).
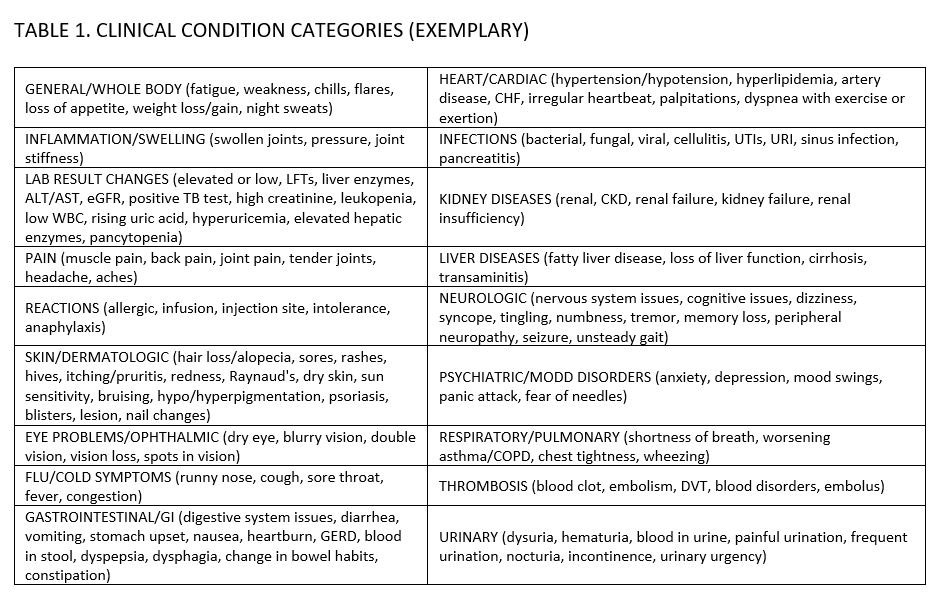
Methods: Data source: PIONEER-Rheumatology, an enhanced database combining fielded EMR data with extracted information from open text (office visits, infusions logs, and provider-patient communications). Discontinuation reasons in visit notes were classified by clinical scribes into categories [FIGURE 1] and supplemented with additional context for greater granularity. Based on scribe input, discontinued episodes were labeled as attrition (death, moved/left practice, non-clinical reasons), lost to follow up (LTFU, patient disengaged from care), or clinical (treatment goal achieved, lack/loss of efficacy, or condition resulting from disease, treatment, or patient burden). Clinical reasons not reflecting lack/loss of efficacy were further stratified into manageable (defined as potentially avoidable or controlled with provider or patient action) v. non-manageable conditions (comorbid conditions, e.g. cancer). Study population: Adult (18+ years old) patients with RA who discontinued one or more b/tsDMARDs episodes between 2017 to 2021. Statistical comparisons made using chi-square (SPSS v22).
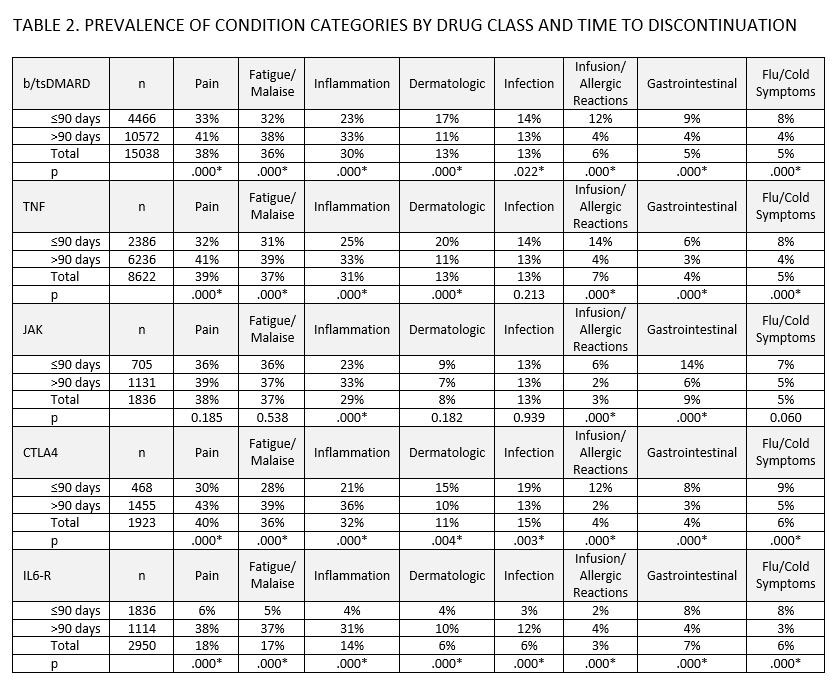
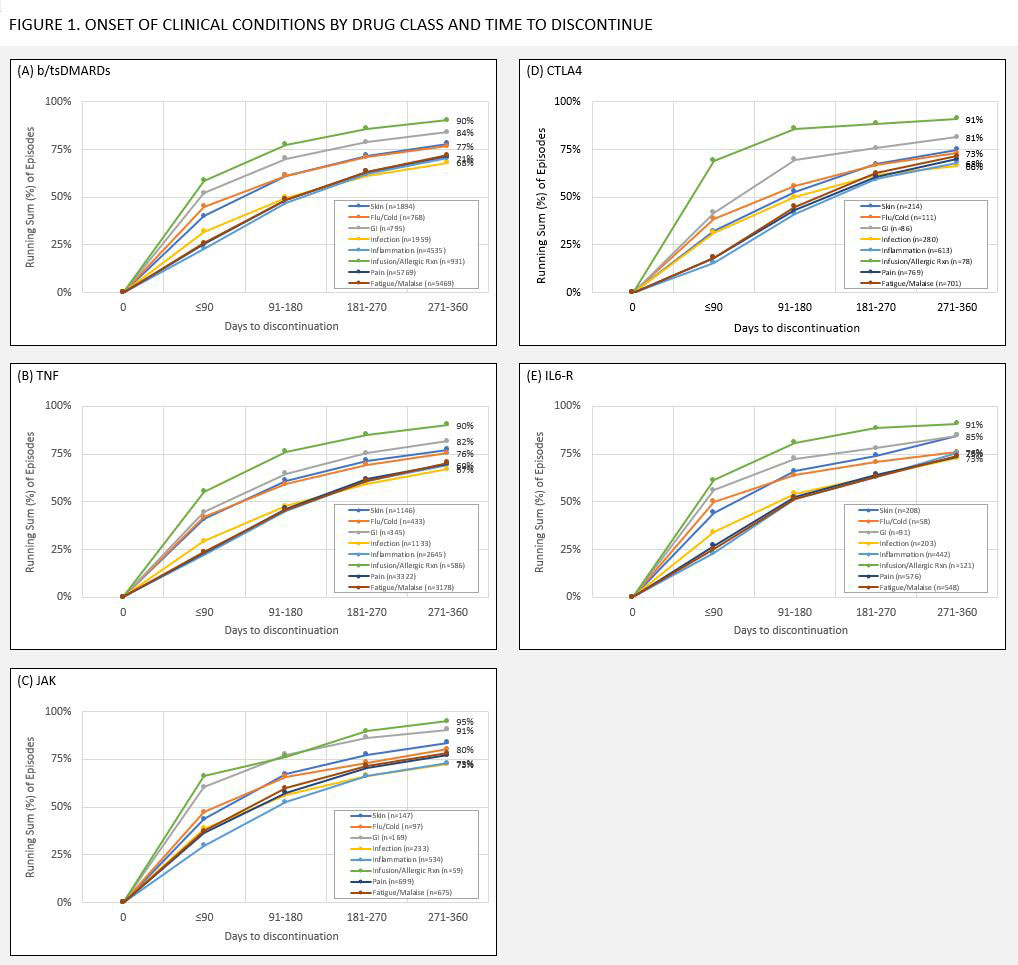
Results: 10328 patients discontinued 20343 b/tsDMARD episodes; LTFU accounted for 5% (1026) of discontinuations, 21% (4279) were due to attrition, and 74% (15038) clinical reasons. Of episodes with 1 or more clinical reason, 2% (296/15038) resulted from achieving treatment goals, 54% (8058/15038) indicated lack of efficacy, 37% (5338/15038) non-manageable condition, and 41% (6136/15038) manageable condition. Most common conditions were pain (38%, 5769/15038), fatigue/malaise (36%, 5469/15038), inflammation (30%, 4535/15038), infection (13%, 1959/15038), dermatologic (13%, 1894/15038), infusion/allergic reaction (6%, 931/15038), gastrointestinal (5%, 795/15038), and flu/cold-like symptoms (5%, 768/15038). For episodes discontinued due to a clinical reason (n=15038), episodes discontinued within 90 days had higher proportions of discontinuations citing dermatologic issues (17% v. 11% post 90 days), infusion/allergic reactions (12% v. 4% post 90 days), gastrointestinal (9% v. 4% post 90 days), and flu/cold-like symptoms (8% v. 4% post 90 days), though proportions and differences varied by drug class. [TABLE 2] The onset of conditions, as a function of time to discontinuation, was similar across drug classes, though observable differences in slope were evident between certain drug classes. [FIGURE 1]
Conclusion: This study of 20343 discontinued b/tsDMARD episodes provides a foundation for understanding the timing of discontinuations relative to reasons for such, and may inform care strategies to enable maximal therapeutic benefit.
To cite this abstract in AMA style:
Huston K, Adams C, Helfgott S, singh J, Soloman N, Persons D, Milligan S, Edgerton C. Reasons for Early Discontinuation of Targeted Synthetic (ts) or Biologic (b) DMARDs; Chart Review of 20,343 Drug Episodes Given to Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/reasons-for-early-discontinuation-of-targeted-synthetic-ts-or-biologic-b-dmards-chart-review-of-20343-drug-episodes-given-to-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/reasons-for-early-discontinuation-of-targeted-synthetic-ts-or-biologic-b-dmards-chart-review-of-20343-drug-episodes-given-to-patients-with-rheumatoid-arthritis/