Session Information
Date: Monday, October 27, 2025
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: SjD is a chronic, systemic, progressive autoimmune disease characterized by dry mouth and eyes, but it is often accompanied by extraglandular symptoms and signs that affect the entire body. About two-thirds of SjD occurs alone; the remaining one-third is associated with other autoimmune connective tissue diseases such as RA, SLE, or systemic sclerosis. This analysis describes treatment patterns and health care resource use (HCRU) over 12 months in US patients newly diagnosed with SjD.
Methods: This retrospective cohort analysis included adult patients with a first diagnosis (index date) of sicca syndrome or SjD (ICD-9-CM: 710.2; ICD-10-CM: M35.0-9, M35A-C; together termed SjD here) from October 2015 through March 2023 (identification period) and a second diagnosis within 28 to 365 days of first diagnosis in the Optum EHR database. Patients had ≥12 months pre-index documented clinical history with no record of a SjD diagnosis (baseline period), as well as ≥12 months follow-up post index (follow-up period). Treatment patterns and HCRU were assessed during the follow-up period. Patients’ HCRU and medication use in the baseline and follow-up periods were reported descriptively.
Results: Of the 35,035 patients with SjD who met the inclusion criteria, 67.2% had SjD only whereas 32.8% had SjD occurring with other autoimmune conditions such as RA, SLE, and systemic sclerosis (SjD+other). HCRU for all patients with SjD increased from the baseline to follow-up period, irrespective of whether patients had SjD only or SjD+other (Table 1). An increase in patients with HCRU from baseline to follow-up was observed across all HCRU categories (all-cause outpatient visits [OP, 89.9% vs 99.6%], inpatient admissions [IP, 5.0% vs 11.8%], emergency department visits [ED, 16.5% vs 20.8%], SjD-specific diagnostic procedures [28.5% vs 41.2%], lab tests [55.2% vs 74.6%], and imaging [26.6% vs 36.8%]). A higher proportion of SjD+other patients had an IP visit in the follow-up period compared with SjD only patients. In addition to the increase in the proportion of patients with any HCRU during the follow-up period, the mean (SD) number of IP, ED, and lab visits per patient per year (PPPY) increased slightly in the follow-period vs baseline, with a substantial increase in OP visits (Table 1). Mean OP visits PPPY increased to a greater extent in the SjD+other group (41% increase) vs the SjD only group (32% increase). The 3 most commonly prescribed treatment classes in the overall cohort were systemic corticosteroids (42.8%), antidepressants (32.2%), and hydroxychloroquine and antimalarials (29.0%) (Table 2). This trend was similar for the SjD+other cohort; however, in the SjD only cohort, NSAIDs were among the top 3 classes along with systemic corticosteroids and antidepressants. Conventional DMARDs and biologic DMARDs were used by 14.2% and 5.6% of patients, respectively, in the follow-up period.
Conclusion: This real-world analysis confirmed that patients with newly diagnosed SjD alone or with other autoimmune diseases have high disease burden within 12 months of diagnosis as demonstrated by increases in HCRU, largely driven by greater use of OP, IP, and ED services. Although treatment use increased during follow-up, biologic use was limited.
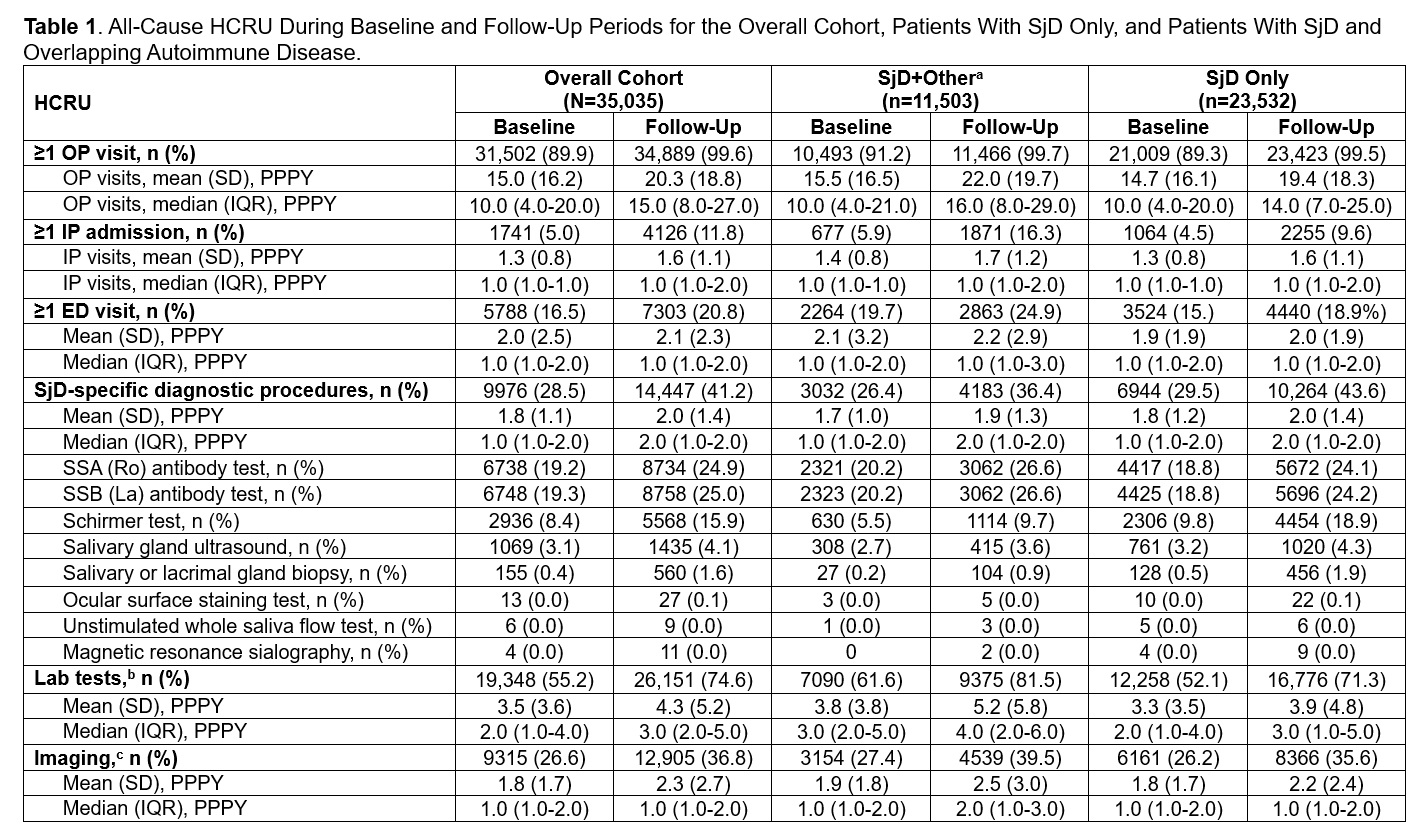 ED, emergency department; IP, inpatient; IQR, interquartile range; OP, outpatient; PPPY, per patient per year; SjD, Sjögren disease; SSA, Sjögren’s-syndrome-related antigen A; SSB, Sjögren’s-syndrome-related antigen B.
ED, emergency department; IP, inpatient; IQR, interquartile range; OP, outpatient; PPPY, per patient per year; SjD, Sjögren disease; SSA, Sjögren’s-syndrome-related antigen A; SSB, Sjögren’s-syndrome-related antigen B.
a Associated autoimmune conditions were looked in all available pre-index and 1 year post-index period.
b Lab tests include the following: parotidectomy, punctal occlusion, antinuclear antibodies, rheumatoid factor, Anti-double stranded DNA (dsDNA%) test (for associated lupus), beta 2 microglobulin, complement C3, complement C4, complete blood count with differential, comprehensive metabolic panel, cryoglobulins, cyclic citrullinated peptide (associated rheumatoid arthritis), free light chain, lactate dehydrogenase, myositis antibodies, pulmonary function test, scleroderma antibodies, serum protein electrophoresis, uric acid, urine protein electrophoresis.
c Imaging includes chest x-rays, computed tomography scans, magnetic resonance imaging, and ultrasound.
.jpg) bDMARDs, biologic disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; NSAIDs, nonsteroidal anti-inflammatory drugs; SjD, Sjögren’s disease; TNFi, tumor necrosis factor inhibitor.
bDMARDs, biologic disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; NSAIDs, nonsteroidal anti-inflammatory drugs; SjD, Sjögren’s disease; TNFi, tumor necrosis factor inhibitor.
a Associated autoimmune conditions were looked in all available pre-index and 1 year post-index period.
To cite this abstract in AMA style:
McCoy S, Lalla A, Choudhary N, Chatterjee S, Prasad S, Egana A. Real World Treatment Patterns and Health Care Resource Use in Patients With Newly Diagnosed Sjögren’s Disease (SjD) in the United States [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-treatment-patterns-and-health-care-resource-use-in-patients-with-newly-diagnosed-sjogrens-disease-sjd-in-the-united-states/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-treatment-patterns-and-health-care-resource-use-in-patients-with-newly-diagnosed-sjogrens-disease-sjd-in-the-united-states/
