Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Nintedanib is an antifibrotic medication used to treat interstitial lung disease (ILD), including connective tissue disease-associated ILD (CTD-ILD). Clinical trials that led to its approval for use in systemic sclerosis-associated ILD in 2019 and its approval for use in progressive fibrosing ILD (including many CTD-ILDs) in 2020 reported moderate rates of gastrointestinal and other side effects. However, there have been limited real-world data on its tolerability. This study seeks to describe and quantify real-world tolerability of nintedanib in CTD-ILD patients treated at an academic tertiary care center.
Methods: In this single-center retrospective chart review, we identified eligible patients via electronic medical record query of ICD-10 diagnostic codes from 2019-2024. Eligible patients were adults who had been diagnosed with ILD by a pulmonologist and an associated connective tissue disease by a rheumatologist, and had been prescribed nintedanib. Due to the retrospective nature of the study and lack of ACR classification criteria for all represented CTD-ILDs, eligibility was determined by clinical diagnosis rather than by classification criteria. Data including demographics, medical history, concurrent medications, nintedanib dose, and reported side effects were manually abstracted from the electronic medical record and were verified by two reviewers. Descriptive statistics were employed.
Results: We identified 33 patients with CTD-ILD who had been prescribed nintedanib (mean age 68 years, 58% female). Underlying autoimmune conditions included rheumatoid arthritis (36%), systemic sclerosis (36%), systemic lupus erythematosus (6%), Sjogren’s disease (6%), overlap syndromes (6%), idiopathic inflammatory myopathy (3%), interstitial pneumonia with autoimmune features (3%), and inflammatory arthritis (3%). Eighty-eight percent of patients were prescribed immunomodulatory therapy concurrently with nintedanib, most commonly mycophenolate (48%), hydroxychloroquine (18%), rituximab (11%), and azathioprine (11%), among others.
The most common side effects reported were diarrhea (42%) and nausea and vomiting (12%). Three patients (9%) developed elevated liver function tests, and one of these patients (3%) developed liver function tests greater than five times the upper limit of normal.
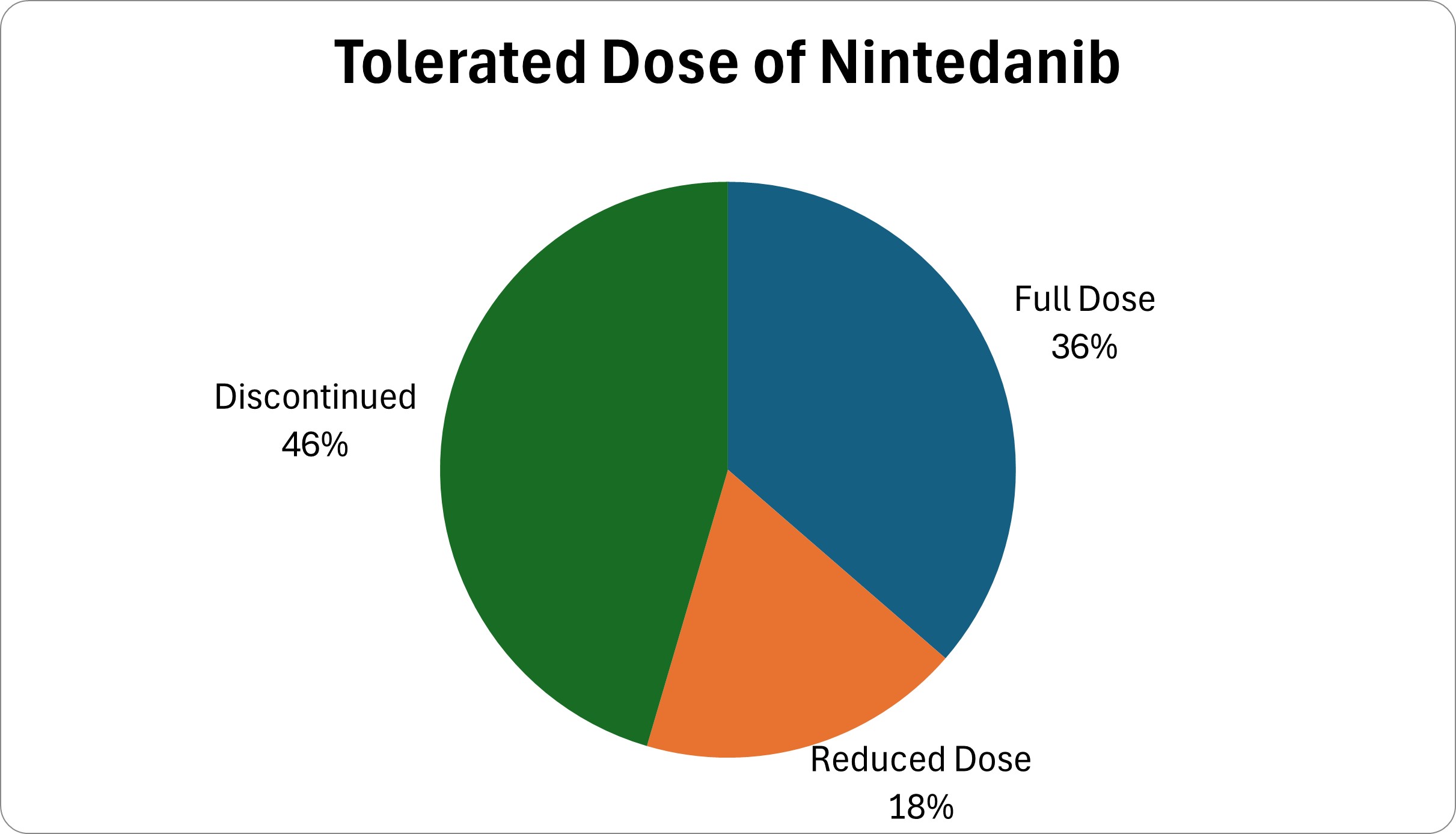
Twelve patients (36%) remained on nintedanib at the standard dose. Six patients (18%) remained on nintedanib at a reduced dose. Fifteen patients (46%) discontinued the medication (Figure 1). The mean duration of nintedanib use was 16.8 months.
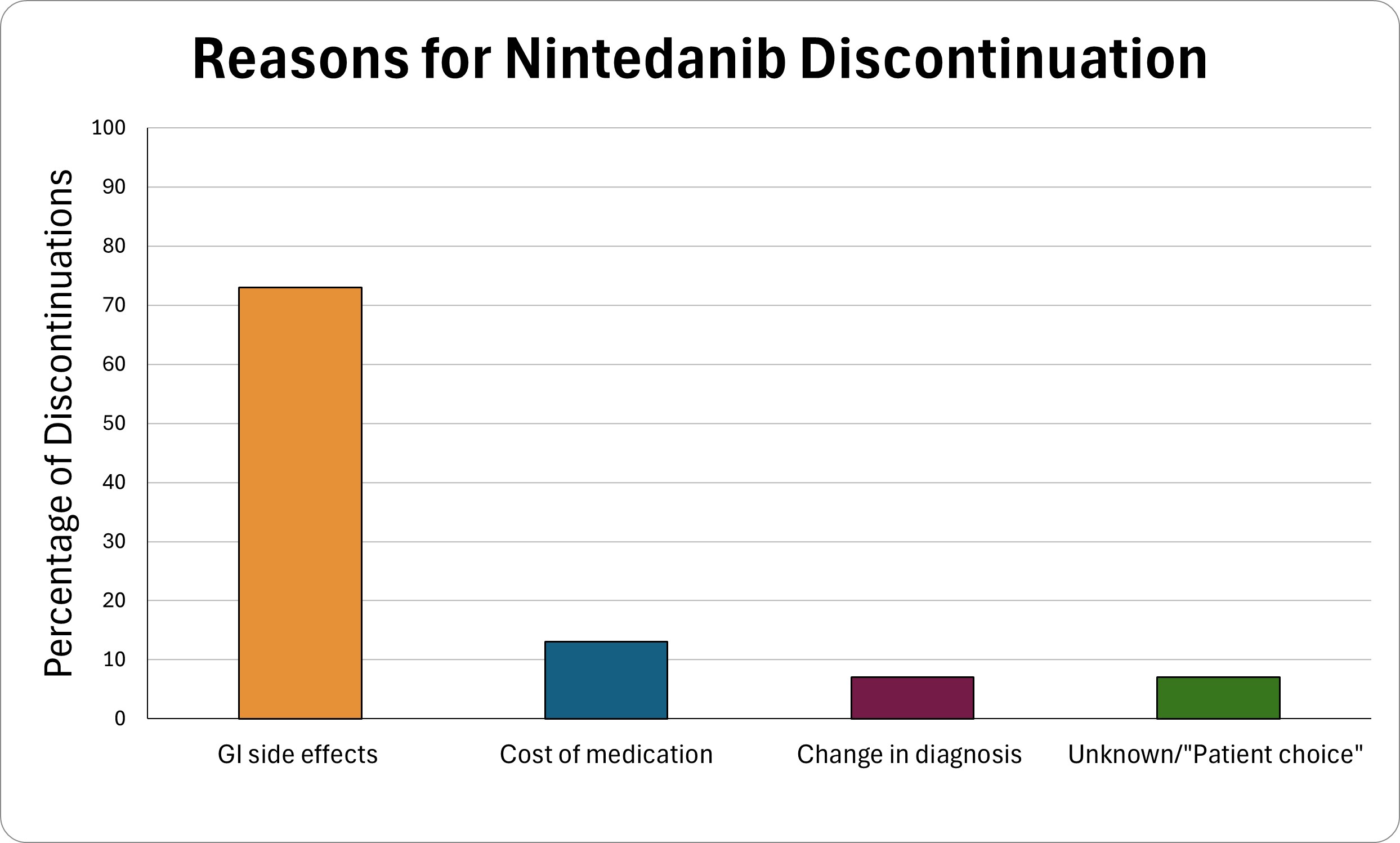
The most common reasons for medication discontinuation were gastrointestinal side effects (73% of discontinuations) and medication cost (13% of discontinuations) (Figure 2).
Conclusion: In our center, nintedanib was prescribed for a wide variety of CTD-ILDs, and was often prescribed concurrently with systemic immunomodulatory therapy. Gastrointestinal side effects were common and were often the reason for medication discontinuation. Due to side effects and medication cost, a minority of patients were able to tolerate the standard dose of nintedanib long-term. Clinicians should consider these limitations when prescribing nintedanib.
To cite this abstract in AMA style:
Goyal S, DiSilvio B, Marco J. Real-World Tolerability of Nintedanib in Connective Tissue Disease-Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-tolerability-of-nintedanib-in-connective-tissue-disease-associated-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-tolerability-of-nintedanib-in-connective-tissue-disease-associated-interstitial-lung-disease/