Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex, chronic autoimmune disease characterized by frequent episodes of disease activity (flares) of varying severity.1 SLE flares are often managed with corticosteroids, the frequent and extended use of which can cause severe side effects.2 Anifrolumab clinical trials have shown reductions in flare rates along with corticosteroid dose tapering – two major treatment goals for SLE.3,4 This study examined real-world changes in disease flares and oral corticosteroid (OCS) use before and after initiation of anifrolumab therapy in adults with SLE in the United States.
Methods: This retrospective observational study included claims data of adults with SLE in the MORE2 Registry® (Commercial, Medicare Advantage, and Managed Medicaid), plus 100% Medicare Fee-for-Service data (8/1/2020-9/30/2023). Eligible patients: 1) had an SLE diagnosis: ≥1 inpatient or ≥2 non-diagnostic outpatient claims with SLE diagnosis code (ICD-10-CM M32.xx) separated by 30-365 days; 2) had initiated anifrolumab: ≥1 claim for anifrolumab on/after August 1, 2021 (earliest claim = index date); 3) were adherent to anifrolumab (≥3 claims in a 6-month period); 4) and ≥18 years old at index, 5) with ≥6 months of continuous enrollment with insurance benefits before and after index. Outcomes were assessed in 6-month windows pre-index (baseline) and post-index (follow-up). Outcomes of interest included: i) SLE disease flares (proportion of patients experiencing flares by frequency [≥1 to ≥4] and severity [moderate, severe]), as determined with an adaptation of the validated algorithm from Garris et al,5 and ii) prednisone-equivalent OCS use (number of claims, mean daily OCS dose, cumulative OCS dose). Group estimates were reported.
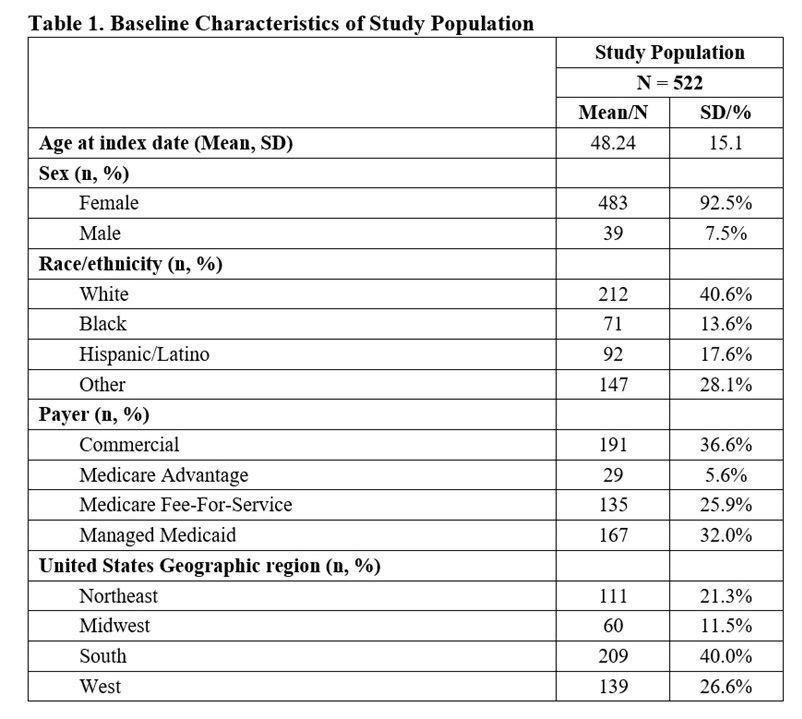
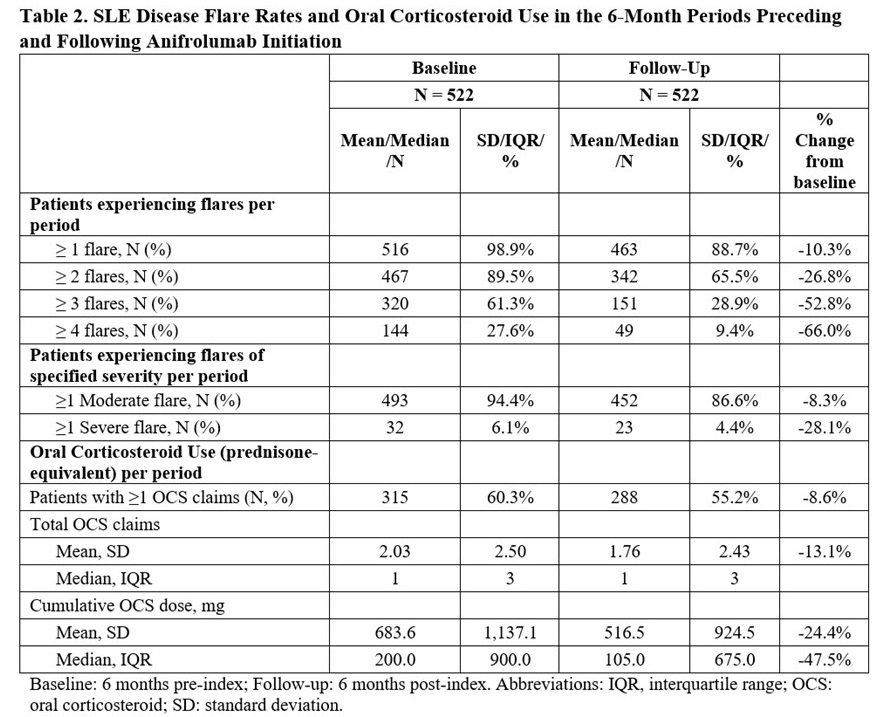
Results: Of the 522 eligible participants, 40.6% were white, 92.5% female, 36.6% commercially insured, with a mean (SD) age of 48.2 (15.1) years (Table 1). At baseline, 516/522 (98.9%) patients experienced ≥1 flare, 467 (89.5%) ≥2 flares, 320 (61.3%) ≥3 flares, and 144 (27.6%) ≥4 flares (Table 2). During follow-up, 463/522 (88.7%) patients experienced ≥1 flare, 342 (65.5%) ≥2 flares, 151 (28.9%) ≥3 flares, and 49 (9.4%) ≥4 flares. The proportion of patients with moderate and severe flares decreased from 94.4% and 6.1% at baseline to 86.6% and 4.4% during follow-up, respectively. The mean (SD) cumulative OCS dose declined by 24% from 684 (1137) mg pre-index to 516 (924) mg post-index (Table 2).
Conclusion: This real-world study showed reductions in the frequency of SLE disease flares and OCS use in the 6 months following anifrolumab treatment initiation. These early changes in disease flare rates and cumulative OCS dose among SLE patients suggest the potential for steroid-sparing with anifrolumab therapy. Further analyses to determine long-term outcomes with anifrolumab are underway.
References:
1. Hammond E, et al. Lupus Sci Med. 2021;8(1):e000504
2. Frodlund M, et al. Rheumatology. 2024;63(4):1104-1112
3. Furie R, et al. Lupus. 2021;30(8):1254-1263
4. Kalunian K, et al. Arthritis Rheumatol. 2023;75(2):253-265
5. Garris C, et al. J Med Econ. 2013;16(5):667-77
To cite this abstract in AMA style:
Binka M, Muhammad Qasim Khan R, Tkacz J, McMorrow D, Gozalo L, Kerr G. Real-World Reduction in Disease Flares and Oral Corticosteroid Use with Anifrolumab Therapy in Systemic Lupus Erythematosus: A Claims-Based Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/real-world-reduction-in-disease-flares-and-oral-corticosteroid-use-with-anifrolumab-therapy-in-systemic-lupus-erythematosus-a-claims-based-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-reduction-in-disease-flares-and-oral-corticosteroid-use-with-anifrolumab-therapy-in-systemic-lupus-erythematosus-a-claims-based-study/