Session Information
Date: Monday, October 27, 2025
Title: (1434–1466) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Real world data from community healthcare systems can provide insights into patient trajectories with psoriasis (PsO) and psoriatic arthritis (PsA): baseline characteristics, patterns of treatment, and transitions between PsO and PsA.
Methods: Patients with a new PsO or PsA diagnosis were identified from 51 hospitals and 1,085 clinics in Providence Health & Services, which serves urban centers and rural catchment areas across seven states in the Western US. The study period was Jan 1, 2011 to Aug 31, 2023. The PsO cohort index time t0 was the first diagnosis of PsO without PsA, and the PsA t0 was the first diagnosis of PsA. Baseline characteristics were determined by the most recent results during the year before t0. Medication use was observed for 6 months after t0. For the PsO cohort, we analyzed time-to-onset of PsA. For the PsA cohort, we analyzed time-to-augmentation and time-to-discontinuation for the first line of systemic immunomodulatory therapy (excluding steroids and NSAIDs). Time-to-event analyses were conducted using Aalen-Johansen survival estimators. Patients were right-censored at the end of their most recent encounter or at the end of the study period, whichever came first, and death considered as a competing outcome.
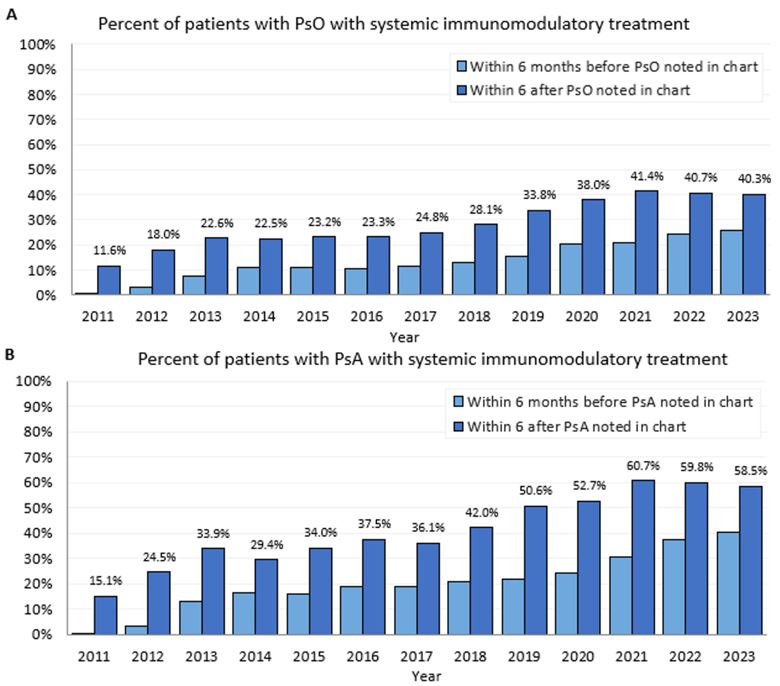
Results: Of 96,533 patients with PsO, 7,558 (7.8%) had PsA at the time PsO was first reported. There were 88,975 patients with PsO without PsA (median age 54, 54.6% female) and 23,083 patients with PsA (median age 56, 59.1% female). See Table 1 for baseline characteristics. Within 6 months after t0, patients use of systemic immunomodulatory medications included the following classes: steroid (PsO 16.7%, PsA 19.9%), antimetabolite (PsO 1.8%, PsA 11.6%), TNF-α inhibitor (PsO 1.5%, PsA 11.6%), calcineurin inhibitor (PsO 1.0%, PsA 0.5%), PDE4 inhibitor (PsO 0.8%, PsA 3.5%), IL-17 inhibitor (PsO 0.6%, PsA 3.3%), IL-23 inhibitors (PsO 0.6%, PsA 0.9%), IL-12/23 inhibitor (PsO 0.5%, PsA 1.2%), JAK inhibitor (PsO 0.1%, PsA 0.9%), and TYK2 inhibitor (PsO 0.0001%, PsA 0.0001%). The percentage of patients with systemic immunomodulatory medications within 6 months after t0 (excluding steroids and NSAIDs) increased from 2011 (PsO 11.6%, PsA 15.1%) to 2023 (PsO 40.3%, PsA 58.5%). Time from PsO to PsA (Figure 1a) and time from the first PsA systemic immunomodulatory line of treatment to discontinuation or augmentation (Figure 1b) are shown below.
Conclusion: Data from a large community health system provides new insight into real world trajectories for patients with PsO and PsA. At the time of first reported PsO diagnosis, many patients have additional chronic medical conditions. The numbers of patients with PsA with systemic immunomodulatory treatment has increased since 2011 but remains lower than reported in studies conducted in academic centers, consistent with the 2016 MAPP survey.
 Data are n (%) or median (IQR). IMID = immune-mediated inflammatory disease. IQR = interquartile range. AI/AN, American Indian and Alaska Native. NHPI, Native Hawaiian and other Pacific Islander. NA = not applicable. For binary variables, p values are calculated by the Fisher’s exact method; for categorical variables, p-values are calculated by the Chi-squared test of independence; for continuous variables, p-values are calculated by the Mann-Whitney U test. *Time of first diagnosis is defined as the first noted date of a patient’s diagnosis or the first time they came to Providence for care. Total patients with Psoriasis diagnosis, excluding patients with pre-existing PsA diagnosis. Diagnoses in EHR may reflect initial misdiagnosis of rheumatoid arthritis prior to diagnosis of PsA.
Data are n (%) or median (IQR). IMID = immune-mediated inflammatory disease. IQR = interquartile range. AI/AN, American Indian and Alaska Native. NHPI, Native Hawaiian and other Pacific Islander. NA = not applicable. For binary variables, p values are calculated by the Fisher’s exact method; for categorical variables, p-values are calculated by the Chi-squared test of independence; for continuous variables, p-values are calculated by the Mann-Whitney U test. *Time of first diagnosis is defined as the first noted date of a patient’s diagnosis or the first time they came to Providence for care. Total patients with Psoriasis diagnosis, excluding patients with pre-existing PsA diagnosis. Diagnoses in EHR may reflect initial misdiagnosis of rheumatoid arthritis prior to diagnosis of PsA.
.jpg) Figure 1. (A) Patients’ systemic immunomodulatory treatment six months prior to the first diagnosis and six months after the first diagnosis. (A) Patients with Psoriasis condition and without pre-existing Psoriatic arthritis (PsA) condition (B) Patients with PsA condition. Systemic immunomodulatory treatment includes medications used for PsO, PsA, but not steroids or NSAIDs. Time of first diagnosis is defined as the first noted date of a patient’s diagnosis within the healthcare system
Figure 1. (A) Patients’ systemic immunomodulatory treatment six months prior to the first diagnosis and six months after the first diagnosis. (A) Patients with Psoriasis condition and without pre-existing Psoriatic arthritis (PsA) condition (B) Patients with PsA condition. Systemic immunomodulatory treatment includes medications used for PsO, PsA, but not steroids or NSAIDs. Time of first diagnosis is defined as the first noted date of a patient’s diagnosis within the healthcare system
.jpg) Figure 2: (A) Cumulative incidence of new Psoriatic Arthritis (PsA) diagnoses over time. The Y-axis represents the cumulative incidence (%) of patients newly diagnosed with PsA, while the X-axis shows follow-up time in years. (B) Time from first line systems immunomodulatory medication (other than steroids or NSAIDS) of patients with PsA diagnoses to discontinuation (blue) or augmentation (orange). The Y-axis represents the cumulative incidence (%), while the X-axis shows follow-up time in years.
Figure 2: (A) Cumulative incidence of new Psoriatic Arthritis (PsA) diagnoses over time. The Y-axis represents the cumulative incidence (%) of patients newly diagnosed with PsA, while the X-axis shows follow-up time in years. (B) Time from first line systems immunomodulatory medication (other than steroids or NSAIDS) of patients with PsA diagnoses to discontinuation (blue) or augmentation (orange). The Y-axis represents the cumulative incidence (%), while the X-axis shows follow-up time in years.
To cite this abstract in AMA style:
Mease P, Wei Q, Huang L, Hawkes J, Liu C, Wu Y, Wang T, Hager A, Hadlock J. Real-world Patient Trajectories in Psoriasis and Psoriatic Arthritis: a Retrospective Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-patient-trajectories-in-psoriasis-and-psoriatic-arthritis-a-retrospective-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-patient-trajectories-in-psoriasis-and-psoriatic-arthritis-a-retrospective-study/
