Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: LN is a severe manifestation of SLE requiring intensive management. We aimed to characterize real-world treatment patterns in newly diagnosed patients with LN within the United States (US), focusing on use of immunosuppressants (ISTs) and advanced therapies including belimumab and voclosporin.
Methods: This retrospective cohort study utilized US healthcare claims data from the IQVIA PharMetrics® Plus database from January 1, 2017 to December 31, 2023. Adults with LN were identified using ICD-10-CM code M32.14, requiring at least one inpatient or two outpatient claims ≥30 days apart between January 1, 2018 to June 30, 2023. The first LN diagnosis was the index date. Patients needed ≥12 months pre-index and ≥6 months post-index continuous enrollment with no prior LN diagnoses. Exclusions included claims for renal transplant, dialysis, end-stage renal disease, malignancy, HIV, hepatitis B/C, and/or tuberculosis. LN treatments (ISTs, systemic glucocorticoids, antimalarials and antihypertensives) were examined, focusing on IST treatment patterns: initiation, switching, discontinuation (≥90-day supply gap), restarting, and duration of use. First and second lines of therapy (LoT) with ISTs were descriptively assessed, along with concomitant use of other LN treatments. Transitions to the second LoT involved adding or switching ISTs in ≤30 days of the first LoT ending or a ≥90-day treatment gap with or without restarting treatment.
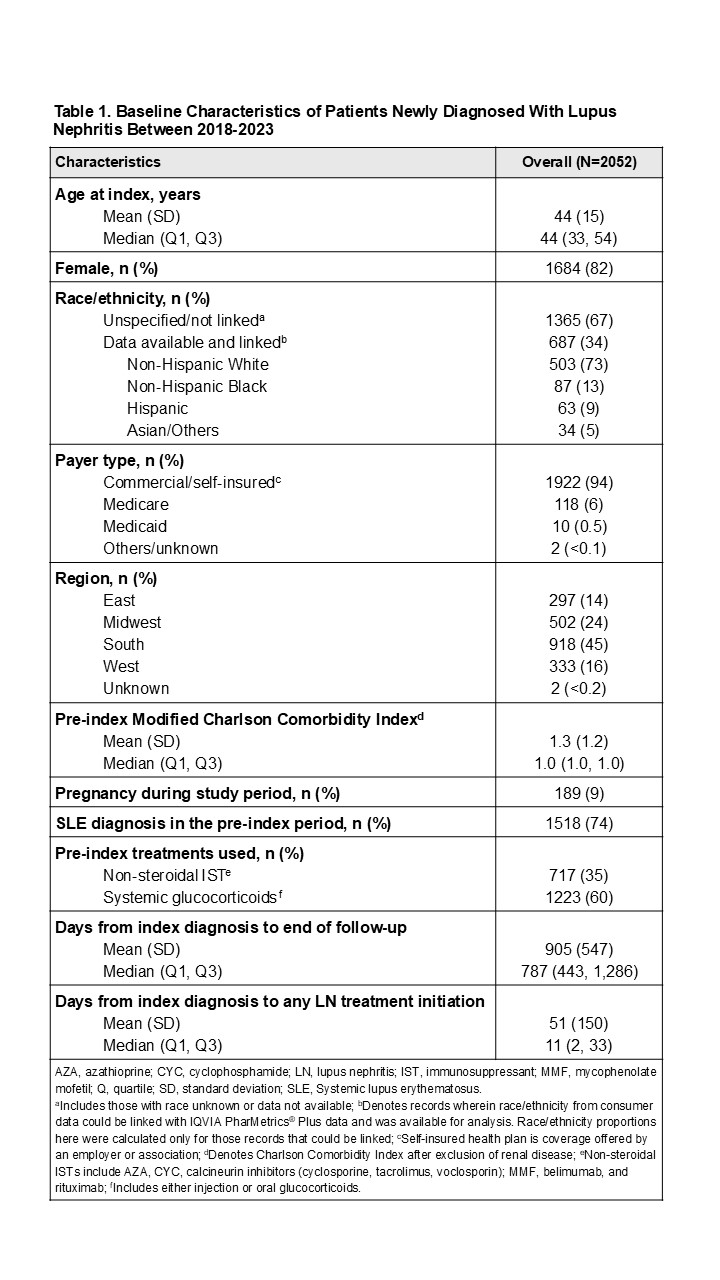
Results: A total of 2052 newly diagnosed patients with LN were identified, with an average follow-up time of 2.5 years. The cohort was 82% female, with a mean (SD) age of 44 (15) years; 94% had private insurance, 74% had a prior SLE diagnosis and 35% had used ISTs pre-index (Table 1). During the post-index period, 1959 patients (95%) received any of the studied LN treatments. Of these, 946 patients (48%) received an IST as their first LoT, primarily mycophenolate mofetil (MMF, 66%), azathioprine (AZA, 10%) and/or belimumab (6%). Median treatment duration (in days) in the follow-up period varied: 257 for MMF, 254 for AZA, and 322 for belimumab. Among patients who received an IST as the first LoT, 80% were given systemic glucocorticoids (oral: 76%, injection: 24%, both: 21%), 65% antimalarials, and 54% antihypertensives (Table 2). By the end of their follow-up, 27% of these patients remained on their initial IST for a median of 456 days, while 19% added or switched to a second LoT with a different IST (primarily MMF, belimumab, or AZA), 16% dropped and restarted an IST after >90 days, and 37% discontinued IST treatment. Additionally, 18% (356/1959) of patients, with any LN treatment post-index, initiated non-IST treatment in the first LoT but switched to an IST during follow-up (Figure 1).
Conclusion: This health insurance claims-based study identified diverse treatment use in newly diagnosed patients with LN, with nearly half receiving an IST in the first LoT. Variability in treatment duration and regimens reflects dynamic patient needs and treatment practices. Understanding these patterns is essential for optimizing LN care and informing future monitoring of treatment changes following practice guideline updates and availability of newer therapies.
To cite this abstract in AMA style:
Patel A, Miller A, Lindsay L, Sheinson D, Xia Z, Pendergraft W, Dall'Era M. Real-World Immunosuppressant Treatment Patterns in Lupus Nephritis: A Retrospective Claims Database Analysis in the United States [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-immunosuppressant-treatment-patterns-in-lupus-nephritis-a-retrospective-claims-database-analysis-in-the-united-states/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-immunosuppressant-treatment-patterns-in-lupus-nephritis-a-retrospective-claims-database-analysis-in-the-united-states/

.jpg)
.jpg)