Session Information
Date: Saturday, November 12, 2022
Title: Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Ankylosing Spondylitis (AS) is an inflammatory rheumatic disease affecting the axial skeleton (1), with a prevalence of up to 0.5% in Europe (2; 3), and occurs in men twice as often than in women (4). 80% develop AS symptoms before the age of 30 (4). Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and biological Disease-Modifying Antirheumatic Drugs (DMARDs) such as Tumour Necrosis Factor inhibitors (TNFi) are the first choice in pharmacological treatments for AS patients (5; 6). Data on disease activity and disease control in AS patients treated in real life settings are limited. Therefore, the main objectives of the study were to evaluate the average disease activity status in the study population and assess the percentage of AS patients with a suboptimal treatment response following at least 12 weeks of treatment with TNFi and/or NSAIDs.
1. Braun, J and J, Sieper. Lancet. 2007, Vol. 369, pp. 1379–90.
2. Braun, J, et al. Arthritis Rheum. 1998, Vol. 41, 1, pp. 58-67.
3. Dean, LE, et al. Rheumatology. 2014, Vol. 53, pp. 650-657.
4. Feldtkeller, E, et al. Rheumatol Int. 2003, Vol. 23, pp. 61–66.
5. Dougados, M, et al. Ann Rheum Dis. 2002, Vol. 61, suppl 3, pp. 40–50.
6. Braun, J, et al. Ann Rheum Dis. 2006, Vol. 65, pp. 316–20.
Methods: INVISIBLE was a multinational, cross-sectional, non-interventional study in Germany, Austria and Belgium. Adult patients with confirmed diagnosis of AS for at least 6 months and ongoing treatment with TNFi and/or NSAIDs for at least 12 weeks prior to enrolment were included. Primary data were collected at a single visit using Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score (ASDAS) and Patient Acceptable Symptom State (PASS) among others. Medical history data were collected from available patient files.
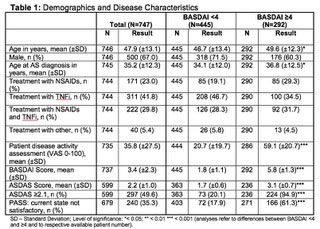
Results: 747 adult AS patients were included in this analysis. 67% of patients were male. Mean symptom duration was 18.2 years. HLA-B27 status was available for 696 patients and 77% of these patients were tested positive. Appr. 23% of patients received NSAIDs, 42% received TNFi, 30% received both NSAIDs and TNFi, 5% received other treatment for at least 12 weeks prior to enrolment. Mean BASDAI and ASDAS scores were 3.4 and 2.2, respectively. Appr. 40% of patients achieved a BASDAI score ≥4 and appr. 50% an ASDAS ≥2.1. Patients with a BASDAI score ≥4 achieved higher scores in most physician and patient reported outcomes. Overall appr. 35% of patients were not satisfied with their current symptom state according to PASS. Of patients with a BASDAI score ≥4 appr. 61% were not satisfied according to PASS.
Conclusion: Results from the non-interventional study INVISIBLE showed that a high proportion of AS patients receiving NSAIDs and/or TNFi have high disease activity according to BASDAI and ASDAS in real life settings. These results suggest that in some patients, disease control and treatment response are suboptimal under current treatment strategy.
To cite this abstract in AMA style:
Brandt-Juergens J, Haschka J, Finsterwalder R, Casier A, Kill A, Dierckx S. Real World Evidence on Disease Activity in Patients with Ankylosing Spondylitis Treated with Tumour Necrosis Factor Inhibitors and/or Nonsteroidal Anti-Inflammatory Drugs in Routine Care [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-on-disease-activity-in-patients-with-ankylosing-spondylitis-treated-with-tumour-necrosis-factor-inhibitors-and-or-nonsteroidal-anti-inflammatory-drugs-in-routine-care/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-on-disease-activity-in-patients-with-ankylosing-spondylitis-treated-with-tumour-necrosis-factor-inhibitors-and-or-nonsteroidal-anti-inflammatory-drugs-in-routine-care/