Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes II: Patient Experience
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Patients with rheumatoid arthritis (RA) have been reported to have higher mortality rates than the general population. Our previous report showed that the standardized mortality ratio (SMR) of Japanese patients with RA registered in the Institute of Rheumatology, Rheumatoid Arthritis (IORRA) cohort between 2000 and 2007 was 1.46-1.90 (Nakajima A. Scand J Rheumatol 2010). Since the introduction of various biological disease-modifying anti-rheumatic drugs (bDMARDs) in Japan after 2007, the mortality rates have declined(Abhishek A. Rheumatology 2018), but it is unclear if the mortality disparity with the general population has decreased. Our study aims to investigate the mortality and its risk factors of Japanese patients with RA since 2007.
Methods: The IORRA cohort is an observational study of patients with RA established in 2000 at Tokyo Women’s Medical University. We observed RA patients who participated in the IORRA survey from October 2007 to September 2020 until October 1, 2021. Since there is no National Death Registry in Japan, we obtained death reports from residual families who responded to our mail query, from affiliated hospitals’ physicians, and from police if a dead patient was found outside of a hospital. The primary endpoint was to calculate SMR using the person-year method with the Japanese general population data as a reference from the Ministry of Health, Labour, and Welfare, Japan. Cases lost to follow-up were treated using multiple imputation methods as a sensitivity analysis of SMR. The secondary endpoints were to examine the risk factors for death using the time-dependent Cox proportional hazards model.
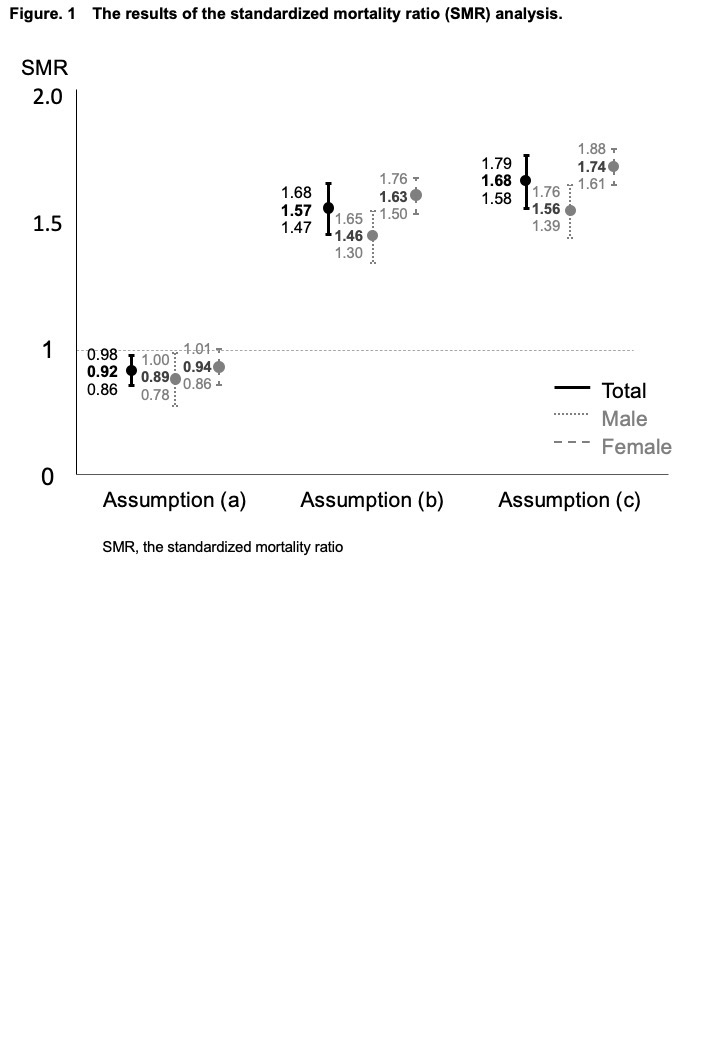
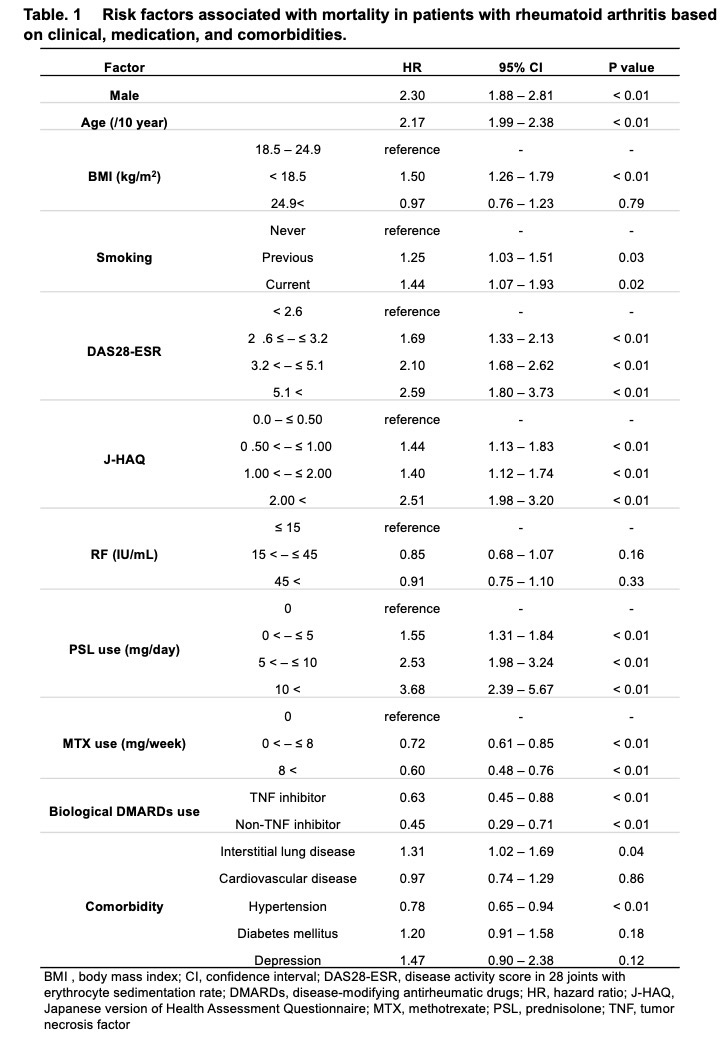
Results: Among 10,613 patients with RA (female, 83.7%; mean age, 57.1±14.1 years; mean disease duration, 9.8±9.6 years; mean observation period, 9.4±4.2 years) who enrolled in the IORRA survey from October 2007 to September 2020, 3,284 (30.9%))were lost to follow up. During the observation period, which covered 99,364.8 person-years, 915 deaths were reported. The leading causes of death were malignancies (28%), respiratory involvement (22%), and cardiovascular disorders (12%). The SMR (95% confidence interval), assuming that all lost-to-follow-up patients were alive, was 0.92 (0.86-0.98). When it was assumed that lost-to-follow-up patients had the same mortality rate as the remaining patients, the SMR was 1.57 (1.47–1.68). Alternatively, when it was assumed that the mortality rate of lost-to-follow-up patients was 1.65 times as high as that of the remaining patients based on the previous report (Kauppi M, J Rheumatol 2005), the SMR was 1.68 (1.58-1.79) (Figure 1). Factors associated with increased mortality included male sex, older age, history of smoking, higher disease activity score in 28 joints (DAS28), higher Japanese version of the health assessment questionnaire (J-HAQ) score, corticosteroid use, and presence of interstitial lung disease. Conversely, factors associated with decreased mortality included methotrexate (MTX) use and bDMARDs use (Table 1).
Conclusion: The mortality in patients with RA has remained consistently poor since 2007 when compared to the general population. MTX and bDMARDs use may reduce the risk of death.
To cite this abstract in AMA style:
Sugitani N, Tanaka E, Inoue E, Abe M, Sugano E, Saka K, Ochiai M, Yamaguchi R, Ikari K, Nakajima A, Yamanaka H, Harigai M. Real-world Evidence for Assessing Mortality Disparity Between the Patients with Rheumatoid Arthritis and the General Population in Japan: Results from the IORRA Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/real-world-evidence-for-assessing-mortality-disparity-between-the-patients-with-rheumatoid-arthritis-and-the-general-population-in-japan-results-from-the-iorra-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evidence-for-assessing-mortality-disparity-between-the-patients-with-rheumatoid-arthritis-and-the-general-population-in-japan-results-from-the-iorra-study/