Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The aim of this study was to evaluate the real-world effectiveness of the disease-modifying treatment belimumab (BEL), comparing the rates of SLE flares among adult patients with SLE in the US who were adherent to BEL over 24 months versus those using standard therapy (ST).
Methods: This retrospective, propensity score-matched cohort study (GSK Study 217377) used medical and pharmacy claims data from the IQVIA PharMetrics Plus database (January 1, 2011–March 31, 2020). Patients with SLE, evidence of intravenous BEL treatment between January 1, 2012 and March 31, 2018 (BEL initiation = index date), and adherence to BEL over 24 months (defined by proportion of days covered ≥80%), were included in the BEL cohort. Patients in the ST cohort comprised those with SLE who were treated with ST (CS, NSAIDs, antimalarials, or immunosuppressants) between January 1, 2012 and March 31, 2018 with ≥2 dispensings/administrations of any ST treatment regimen ≥6 months apart over 24 months; the index date for the ST cohort was randomly selected among ST treatment initiation dates. The baseline period was defined as 6 months prior to the index date. The primary study outcome was the rate of SLE flares (per person-year [PPY]) over 24 months. Patients in the BEL cohort were matched (1:2) with those in the ST cohort; multivariable regression models further adjusted for remaining differences in baseline ST medication use, proportion of patients with ≥1 flare, and inpatient/emergency department visit costs, between matched cohorts (i.e., doubly robust approach). Confidence intervals (CIs) and p-values were calculated using non-parametric bootstrap procedures.
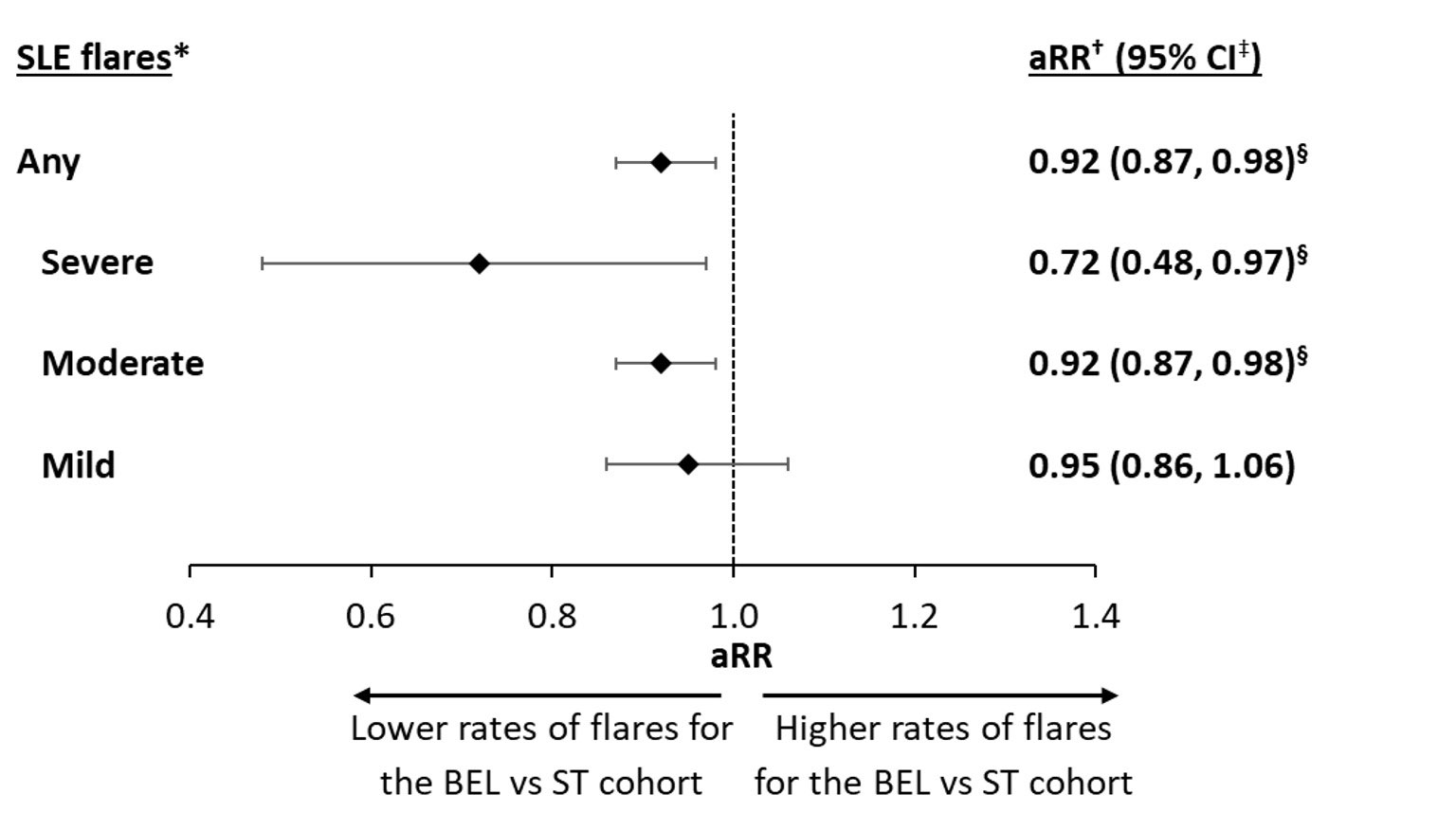
Results: Overall, 364 and 728 patients were included in the BEL and ST matched cohorts, respectively. Baseline characteristics were comparable between the two cohorts; the mean age was similar at 45 years, and 94.2% of patients in both cohorts were female. Overall, 64.6% of the BEL cohort and 63.2% of the ST cohort had moderate SLE disease at baseline, and the majority of patients (BEL: 91.8%; ST: 95.7%) experienced ≥1 flare during the 6-month baseline period. Over the 24-month follow-up period, patients in the BEL cohort had significantly lower rates of SLE flares (of any severity) than those in the ST cohort (adjusted rate ratio, aRR [95% CI]: 0.92 [0.87, 0.98]; p=0.004; Figure). The BEL cohort also experienced significantly lower rates of moderate flares (aRR [95% CI]: 0.92 [0.87, 0.98]; p=0.020) and severe flares (aRR [95% CI]: 0.72 [0.48, 0.97]; p=0.036), translating to 8% fewer moderate flares and 28% fewer severe flares PPY for the BEL cohort compared with the ST cohort. BEL-treated patients also experienced lower rates of mild flares than ST-treated patients, but this difference was not statistically significant (Figure).
Conclusion: This real-world study of claims data showed that adherence to BEL is associated with significantly lower rates of SLE flares relative to ST treatment.
Funding: GSK
*SLE flare episodes were identified using the Garris algorithm [1]; †adjusted rate ratios were calculated from Poisson regression models; ‡95% CI and p-values were generated using non-parametric bootstrap procedures with 499 replications; §statistically significant at the 5% level.
[1] Garris C, et al. J Med Econ. 2013;16:667–77
To cite this abstract in AMA style:
Bell C, Rubin B, Worley K, Germain G, Laliberté F, MacKnight S, Urosevic A, Duh M. Real-World Effectiveness of Belimumab in Patients with SLE in the United States [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-belimumab-in-patients-with-sle-in-the-united-states/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-belimumab-in-patients-with-sle-in-the-united-states/