Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) are the most common types of anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV). Treatment-related toxicities, particularly from glucocorticoid (GC) exposure, are common in patients with AAV. Avacopan is an adjunctive treatment for adults with severe active GPA/MPA that can aid in achieving and sustaining remission while reducing GC use. This study aims to describe patient characteristics, real-world treatment patterns, and outcomes among adults receiving avacopan in the United States.
Methods: This retrospective analysis included administrative claims from the Medicare Fee-for-Service (FFS) and MORE2 Registry® databases from 10/07/2021 to 12/31/2022 (FFS) or 06/30/2023 (MORE2). Adults with ≥1 claim for avacopan (index date: first avacopan claim), continuous enrollment for 12 months prior (baseline) and 12 months after (follow-up) index date were included. Information on demographics, comorbidity burden, including Deyo-Charlson Comorbidity Index (DCI), were collected. Among patients with ≥2 avacopan claims during follow-up, avacopan-specific treatment patterns (i.e., prescriber specialty, median proportion of days covered [PDC], and adherence) were reported. In patients who did not discontinue avacopan (discontinuation defined as ≥90-day gap in treatment) during follow-up, information on GC and other non-GC AAV treatments, percent change in in prednisone-equivalent daily dose (PEDD), and renal and extrarenal manifestations were assessed. Additionally, relapses based on an adapted claims-based algorithm: ≥1 inpatient, ≥1 ER or ≥2 outpatient claims for GPA/MPA, or ≥1 renal or extrarenal claim, each accompanied by >20% increase in PEDD within 30 days, were measured.
Results: A total of 167 patients were included. Mean age was 54.5 years, most were female (57.5%) and White (49.1%), with a median DCI of 2.0 (Table 1). Among patients with ≥2 claims of avacopan (n = 152), the most common specialists to initially prescribe avacopan were rheumatologists (34.9%) and nephrologists (13.2%). The median PDC was 0.8, and over half (53.9%) had a PDC >0.8 at 12 months (Table 2). Nearly half (48.9%) of patients persistent on avacopan treatment (n=94) had chronic kidney disease. Extrarenal manifestations observed during baseline and follow-up included ear, nose and throat, 36.2% and 23.4%, respectively, and chest, 52.1% and 40.4%, respectively (Table 3). These patients also had a median percent change in PEDD of 12.5% between 90 days pre- and 90 days post-index, and 18.3% experienced a relapse during follow-up (Table 3).
Conclusion: Among real-world patients prescribed avacopan early after its availability in the US, demographic and disease characteristics were consistent with the known epidemiologic profile of GPA/MPA. During the 12-month follow-up, most patients showed adequate adherence and did not experience a disease relapse. A reduction in PEDD was observed among patients taking avacopan. Additional studies with longer follow-up are needed to understand the long-term outcomes of this population.
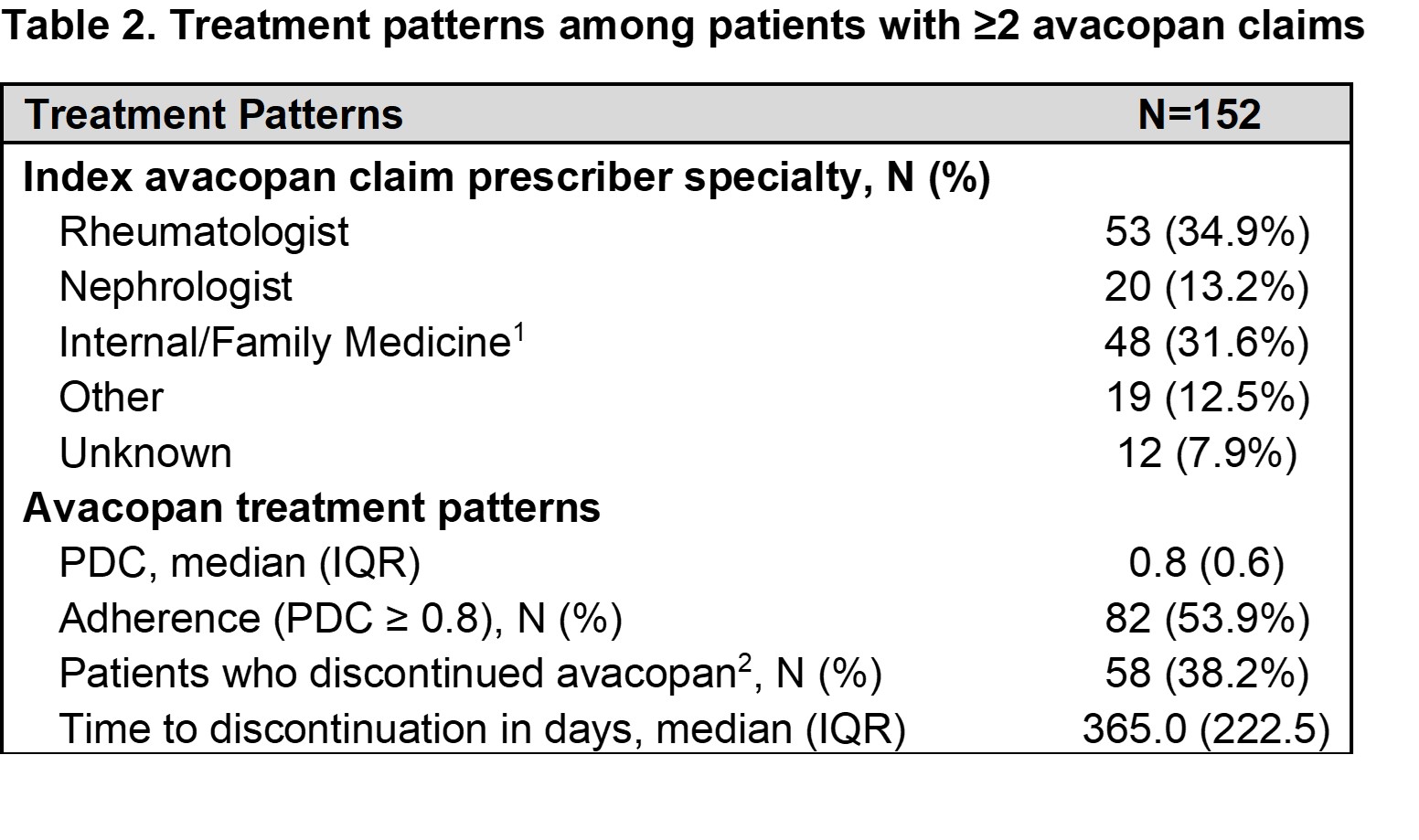 IQR: interquartile range; SD: standard deviation;
IQR: interquartile range; SD: standard deviation;
1. Includes heart failure, cardiovascular disease, cardiomyopathy, myocarditis and myocardial infarction.
.jpg) IQR: interquartile range; PDC: proportion days covered;
IQR: interquartile range; PDC: proportion days covered;
1. Includes physician assistants supervised by a specialist.;
2. Defined as ≥90-day gap in avacopan treatment.
.jpg) GC: glucocorticoid; GPA/MPA: granulomatosis with polyangiitis or microscopic polyangiitis;
GC: glucocorticoid; GPA/MPA: granulomatosis with polyangiitis or microscopic polyangiitis;
1. Includes interstitial lung disease, respiratory failure, pleurisy and alveolar hemorrhage.
To cite this abstract in AMA style:
Sattui S, Ibiloye E, Kathe N, Oh S, Noxon-Wood V, Mohammadi I, Moore-Schiltz L, Li T. Real-World Avacopan Use in Granulomatosis with Polyangiitis and Microscopic Polyangiitis: Insights from United States Claims Data on Outcomes and Adherence [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-avacopan-use-in-granulomatosis-with-polyangiitis-and-microscopic-polyangiitis-insights-from-united-states-claims-data-on-outcomes-and-adherence/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-avacopan-use-in-granulomatosis-with-polyangiitis-and-microscopic-polyangiitis-insights-from-united-states-claims-data-on-outcomes-and-adherence/
