Session Information
Date: Thursday, March 30, 2023
Title: Plenary Abstracts Session I
Session Type: Plenary Session
Session Time: 2:30PM-3:30PM
Background/Purpose: Juvenile-onset systemic lupus erythematosus (JSLE) is associated with chronic inflammation and increased risk of atherosclerosis. The APPLE trial was a randomised, placebo-controlled trial of atorvastatin for atherosclerosis progression in JSLE, using carotid intima-media thickness (CIMT) measurements as primary outcome.
Methods: Unsupervised clustering analysis was used to stratify JSLE patients by their baseline CIMT and identify patterns of CIMT progression over 36 months. An additional in-depth metabolomic analysis was performed to identify lipidomic signatures predictive of CIMT progression. Correlation and univariate regression analyses explored associations between patient and disease characteristics and serum biomarkers. Machine learning techniques and ROC analyses were used to identify and validate a serum metabolomic signature of high CIMT progression.
Results: Baseline CIMT measurements stratified 151 JSLE patients recruited to the APPLE trial into three groups with distinct CIMT progression trajectories irrespective of the treatment allocation (Figure). Two distinct CIMT progression rates (high vs. low), characterised by higher total and low-density lipoprotein (LDL) cholesterol levels (P=0.001 and P=0.002, respectively) were found in the placebo group, while patients treated with atorvastatin had three distinct CIMT trajectories (high, intermediate and low progression), not associated with any relevant biomarkers. A robust metabolomic signature predictive of high CIMT progression in the placebo arm was identified (AUC = 80.7%).
Conclusion: This complementary analysis of the APPLE trial provides new evidence for the significant heterogeneity of subclinical atherosclerosis in JSLE and its distinct progression trajectories irrespective of treatment allocation. Clinical trial patient stratification using the newly identified metabolomic signature predictive of increased natural atherosclerosis progression rate may improve results. Despite being effective in lowering serum lipids, atorvastatin did not prevent the CIMT progression in many at risk JSLE patients, highlighting the need for personalised therapies to address various molecular mechanism driving atherosclerosis in JSLE.
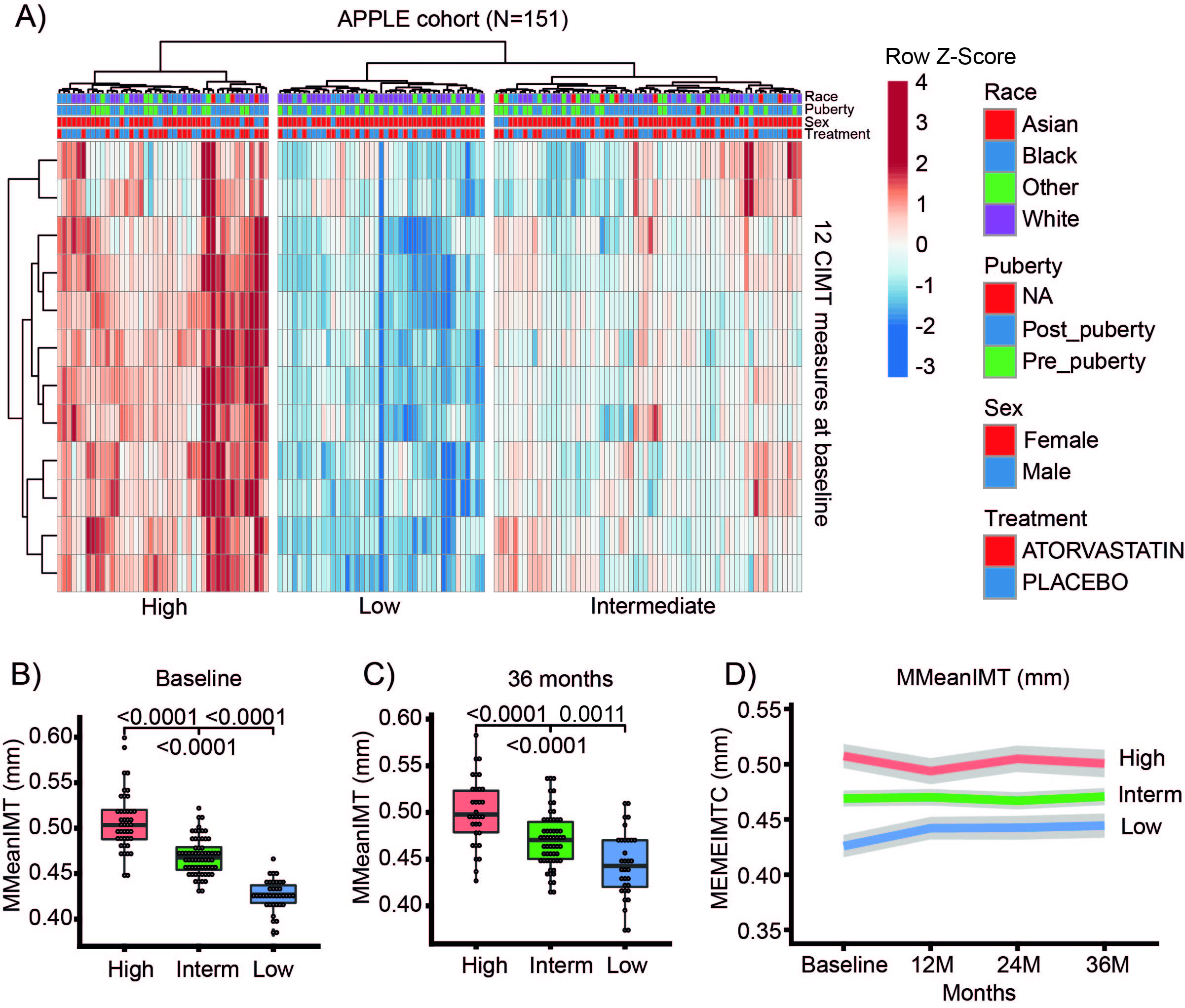 Figure 1: JSLE stratification (all APPLE patients with complete baseline data, Nf151) by baseline CIMT (12 measures). A) Baseline CIMT measures of patients with juvenile-onset SLE were stratified using unsupervised hierarchical clustering. All 12 CIMT measures were standardised within each row by Z score and plotted as a heat map, representing the relationship to the mean of the group (red represents relatively high CIMT measures and blue represents relatively low CIMT measures). Each column represents a patient with JSLE. Three groups of patients with distinct baseline CIMT profiles were identified. B-C) Box and whisker plots show baseline and 36-month MMeanIMT measurements (APPLE primary outcome) in the identified high, intermediate and low baseline CIMT groups. Comparisons between groups were performed using Wilcoxon signed-rank test (* p < 0.05; ** p < 0.01; *** p < 0.001). D) Distinct longitudinal MMeanIMT progression from baseline to 36 months of the high, intermediate and low CIMT progression groups (Mean, 95% CI), irrespective of treatment allocation. (Only JSLE patients with completed CIMT data at 36 months were included in the panel C-D, Nf121). Legend: CIMT- carotid intima-media thickness; MMeanIMT - Mean-Mean IMT common carotid artery measurement.
Figure 1: JSLE stratification (all APPLE patients with complete baseline data, Nf151) by baseline CIMT (12 measures). A) Baseline CIMT measures of patients with juvenile-onset SLE were stratified using unsupervised hierarchical clustering. All 12 CIMT measures were standardised within each row by Z score and plotted as a heat map, representing the relationship to the mean of the group (red represents relatively high CIMT measures and blue represents relatively low CIMT measures). Each column represents a patient with JSLE. Three groups of patients with distinct baseline CIMT profiles were identified. B-C) Box and whisker plots show baseline and 36-month MMeanIMT measurements (APPLE primary outcome) in the identified high, intermediate and low baseline CIMT groups. Comparisons between groups were performed using Wilcoxon signed-rank test (* p < 0.05; ** p < 0.01; *** p < 0.001). D) Distinct longitudinal MMeanIMT progression from baseline to 36 months of the high, intermediate and low CIMT progression groups (Mean, 95% CI), irrespective of treatment allocation. (Only JSLE patients with completed CIMT data at 36 months were included in the panel C-D, Nf121). Legend: CIMT- carotid intima-media thickness; MMeanIMT - Mean-Mean IMT common carotid artery measurement.
To cite this abstract in AMA style:
Ciurtin C, Peng J, Donnes P, Ardoin S, Schanberg L, Lewandowski L, Robinson G, Jury E. Re-analysis of the APPLE (Atherosclerosis Prevention in Paediatric Lupus Erythematosus) Trial Identifies Novel Determinants of Patient Heterogeneity and a Distinct Lipid Metabolomic Signature of Atherosclerosis Progression [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/re-analysis-of-the-apple-atherosclerosis-prevention-in-paediatric-lupus-erythematosus-trial-identifies-novel-determinants-of-patient-heterogeneity-and-a-distinct-lipid-metabolomic-signature-of-ather/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/re-analysis-of-the-apple-atherosclerosis-prevention-in-paediatric-lupus-erythematosus-trial-identifies-novel-determinants-of-patient-heterogeneity-and-a-distinct-lipid-metabolomic-signature-of-ather/
