Session Information
Date: Sunday, November 17, 2024
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Pain is a crucial symptom for patients with RA; early and effective treatment can help alleviate it.1 In this post hoc analysis, we investigated if clinically meaningful pain improvements were associated with greater improvements in other patient-reported outcomes (PROs) in patients with inadequate response (IR) to MTX or biologic DMARDs (bDMARDs), following treatment with upadacitinib 15 mg (UPA15), an oral JAK inhibitor.
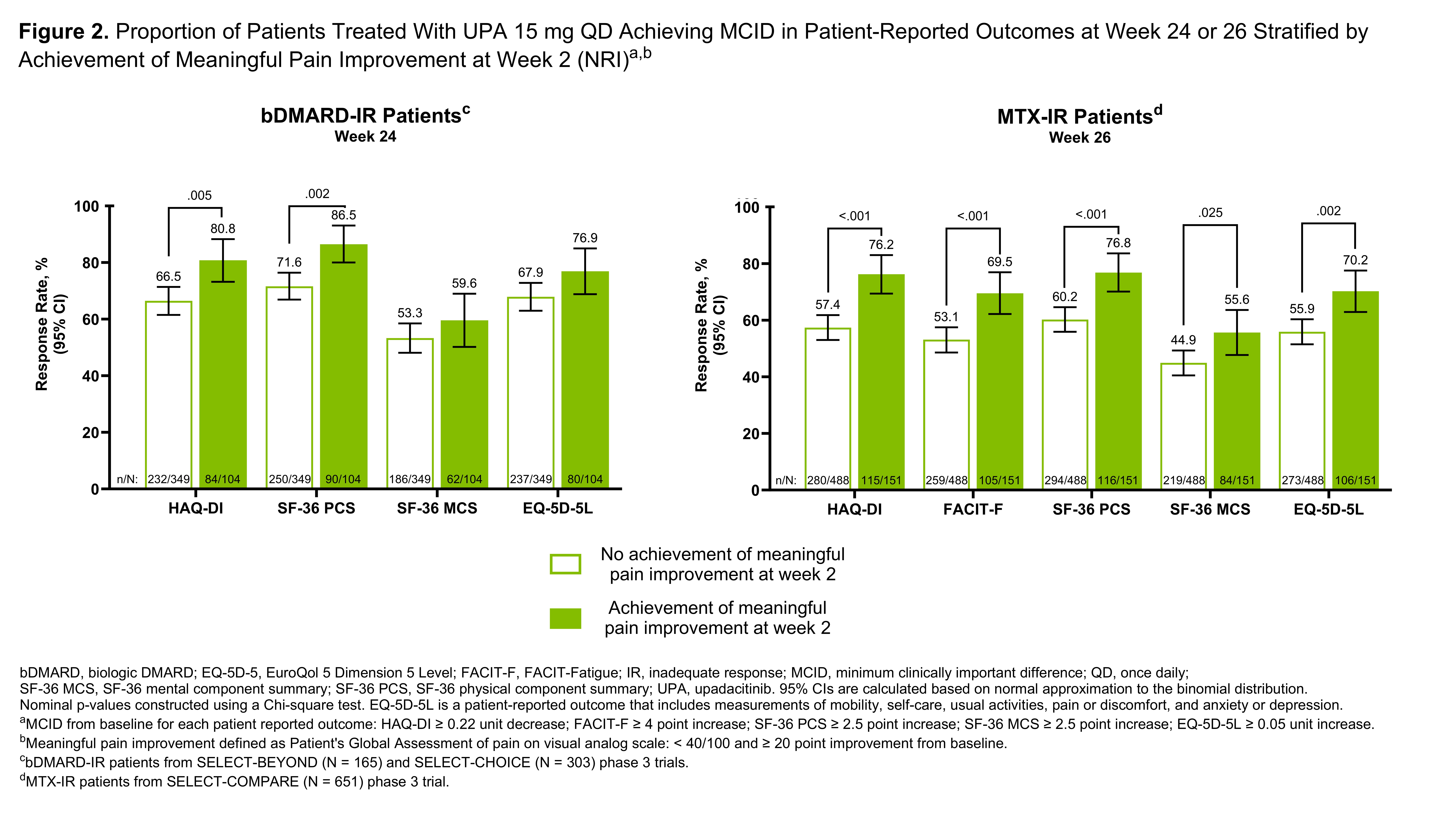
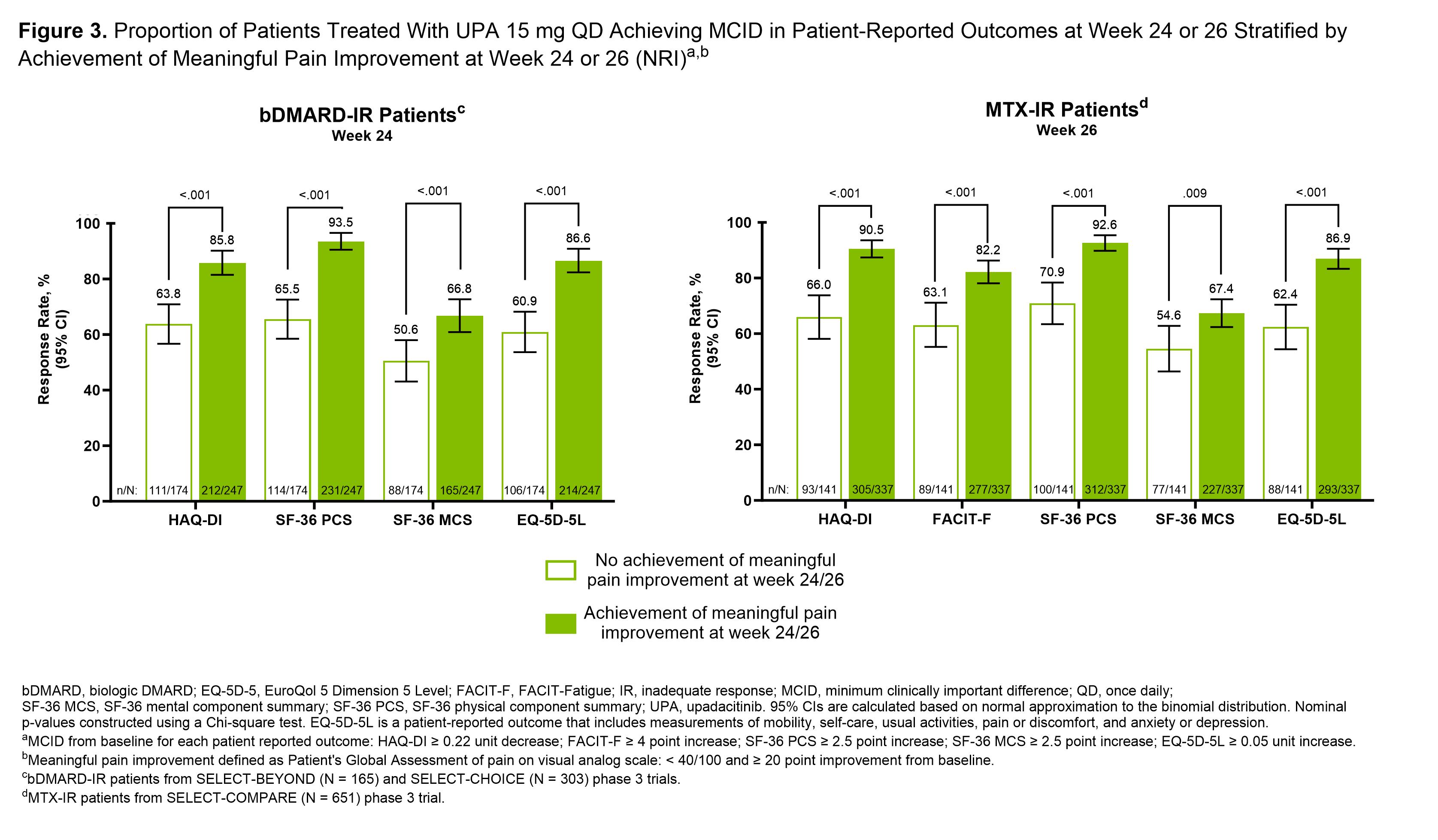
Methods: MTX-IR (SELECT-COMPARE, N=651) and bDMARD-IR patients (SELECT-BEYOND, N=165 and SELECT-CHOICE, N=303) treated with UPA15 were evaluated separately.2,3,4 Proportions of patients achieving clinically meaningful improvement in Patient’s Global Assessment of pain (visual analog scale < 40/100 and ≥20-point improvement from baseline5) were calculated by observed case analysis through wk 24 (bDMARD-IR) or wk 26 (MTX-IR). Patients were stratified by achievement of clinically meaningful pain improvement at two separate timepoints: wk 2 and wk 24 (bDMARD-IR) or wk 26 (MTX-IR). Proportions of patients in each group that achieved minimum clinically important difference (MCID) in HAQ-DI (≥0.22 decrease), FACIT-Fatigue (≥4 increase; MTX-IR only), SF-36 physical component summary (SF-36 PCS, ≥2.5 increase), SF-36 mental component summary (≥2.5 increase), and EuroQol 5 Dimension 5 Level (≥0.05 increase) at wk 24/26 were calculated with nonresponder imputation. Between group differences in the proportion of MCID achievement for each PRO were calculated with nominal P values generated by Chi-square test.
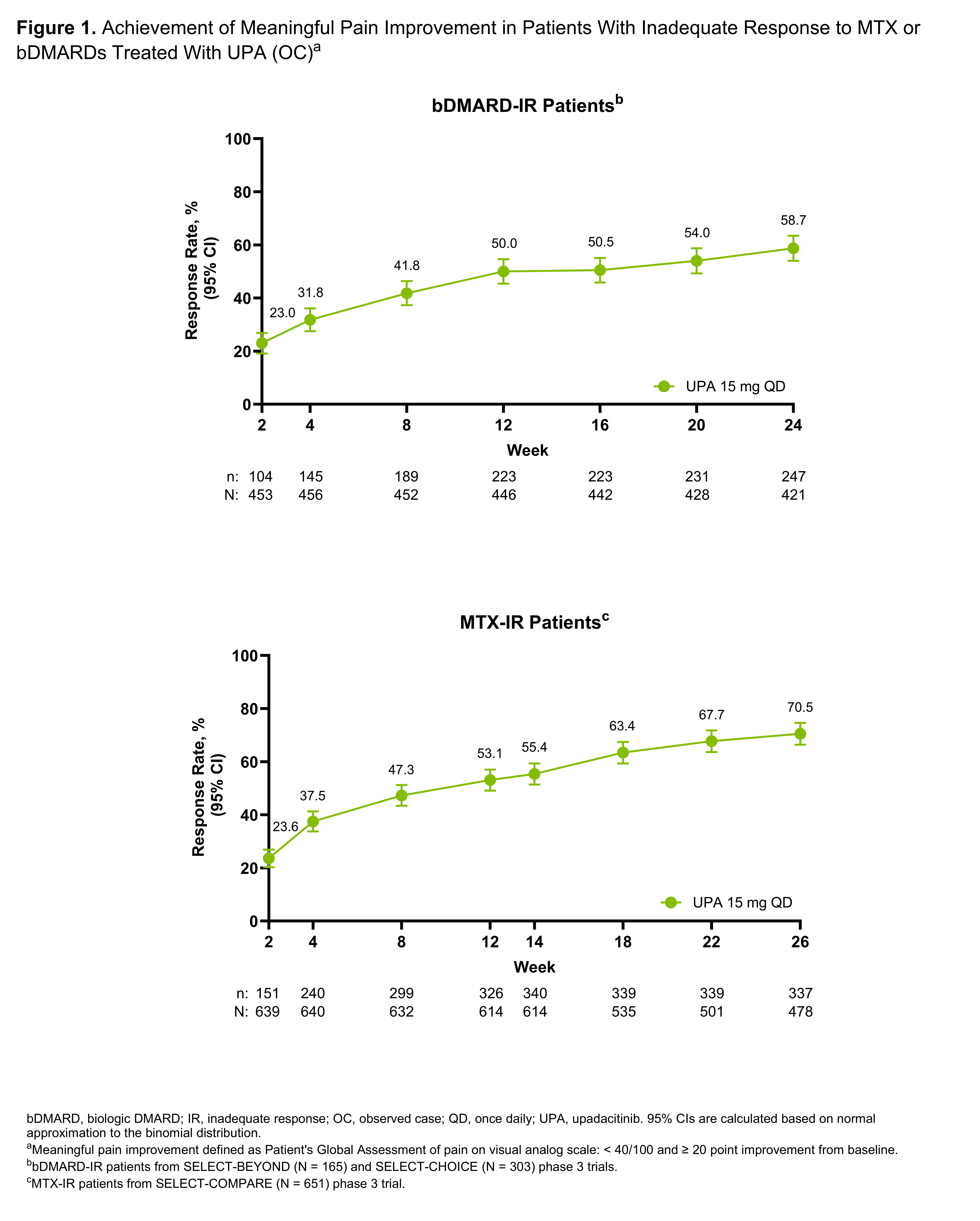
Results: Pain improved rapidly with UPA15 treatment; 23% of bDMARD-IR and 24% of MTX-IR patients achieved clinically meaningful pain improvement at wk 2 (Figure 1). Pain improvement increased over time; 59% of bDMARD-IR and 71% of MTX-IR patients achieved clinically meaningful pain improvement by wk 24/26. In patients who achieved clinically meaningful pain improvement by wk 2, 83% of bDMARD-IR and 90% of MTX-IR patients maintained the response at wk 24/26. For bDMARD-IR patients who achieved meaningful pain improvement at wk 2 compared to those who did not, a larger proportion of patients reported MCID in HAQ-DI and SF-36 PCS at wk 24 (Figure 2). For MTX-IR patients who achieved meaningful pain improvement at wk 2 compared to those who did not, a greater proportion of patients reported MCID in all PROs at wk 26. For both bDMARD-IR and MTX-IR patients who achieved meaningful pain improvement at wk 24/26 compared to those who did not, a larger proportion of patients reported MCID in all PROs at wk 24/26 (Figure 3).
Conclusion: Clinically meaningful pain improvements, as early as wk 2, with UPA15 treatment were associated with more patients reporting achievement of MCID in PROs measuring physical function, fatigue, mental health, and health-related quality of life. Overall, these results suggest that effective treatment of pain is strongly correlated with meaningful improvements in other PROs in RA.
1. Taylor PC. Lancet Rheumatol. 2023;5(6):e351-60.
2. Fleischmann R, et al. Arthritis Rheumatol. 2019;71(11):1788-00.
3. Genovese MC, et al. Lancet. 2022;391(10139):2513-24.
4. Rubbert-Roth A, et al. NEJM. 2020;383(16):1511-21.
5. Tubach F, et al. Arthritis Care Res. 2012;64(11):1699-07.
To cite this abstract in AMA style:
Jain M, Kavanaugh A, Crowley A, Joshi A, Zueger P, Caballero D, Garrison A, Taylor P. Rapid, Clinically Meaningful Pain Improvements Are Associated with Improvements in Other Patient-Reported Outcomes in RA Patients Treated with Upadacitinib [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/rapid-clinically-meaningful-pain-improvements-are-associated-with-improvements-in-other-patient-reported-outcomes-in-ra-patients-treated-with-upadacitinib/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-clinically-meaningful-pain-improvements-are-associated-with-improvements-in-other-patient-reported-outcomes-in-ra-patients-treated-with-upadacitinib/