Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: LEVI-04 is a first-in-class fusion protein (p75NTR-Fc) that supplements the endogenous p75NTR binding protein, providing analgesia via inhibition of NT-3 activity. Like p75NTR, LEVI-04 binds all the neurotrophins (NTs) with differing affinities, with highest to NT-3 and lowest affinity and reversibly to NGF, distinguishing the LEVI-04 mechanism of action from that of anti-NGF antibodies. As serious joint adverse events were seen in the anti-NGF trials, rigorous surveillance of joint safety was performed in this study. In order to properly categorise the risk of adverse joint events with LEVI-04, participants with potentially confounding findings at screening were excluded. LEVI-04 was well tolerated, with no increased incidence of joint pathologies compared to placebo.1
Methods: This was a phase II multicentre (Europe and Hong Kong) RCT in adults with knee OA. Participants were randomized to 4-weekly IV placebo or 0.3, 1, or 2 mg/kg LEVI-04 through week 16, with the final visit at week 20 and a telephone safety follow-up at week 30. Participants who met initial clinical inclusion criteria underwent X-rays of bilateral shoulders, hips and knees, and then MRI of both knees (in some cases, MRI was performed in parallel with X-rays). All images were read centrally and assessed for eligibility. At week 20, all X-rays were repeated, and MRI of the target knee was performed.
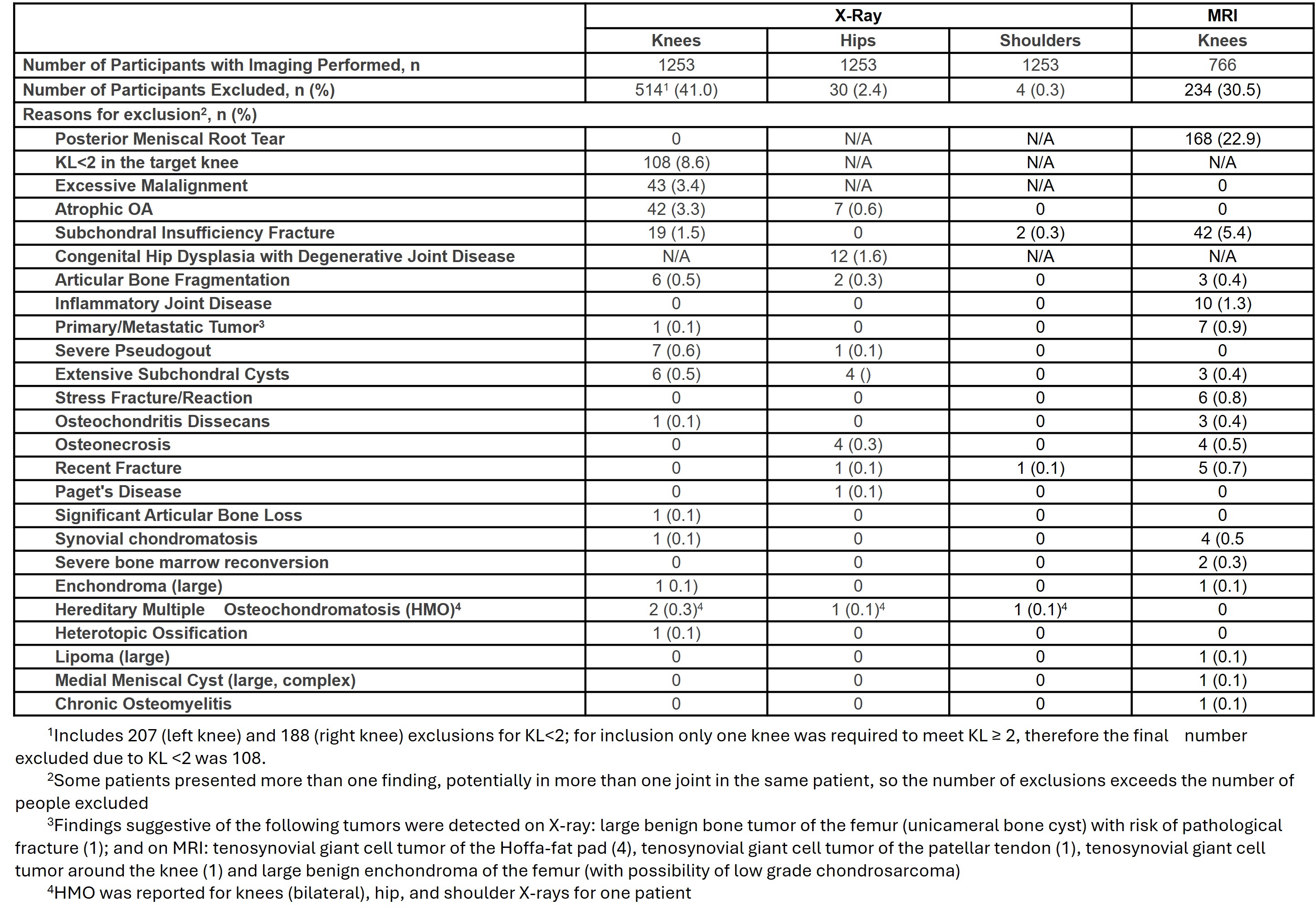
Results: 1598 people with painful knees were screened and 518 participants enrolled. 1080 people (86%) did not proceed past screening. 345 people exited the study before X-rays were performed (151 due to not meeting initial minimum pain in at least one knee, others due to other entry criteria, or sponsor, investigator or participant decision), such that a total of 1253 participants had X-rays of the large joints (Table 1). 514 (41%) people had knee exclusion criteria on X-ray, however this included 207 (left) and 188 (right) knees of KL grade< 2. Only one knee was required to have KL grade >2, resulting in 108 (8.6%) people failing on KL grade. Excessive malalignment and atrophic OA were the next highest criteria, with 43 (3.4%) and 42 (3.3%) failures respectively. 766 people proceeded to MRI of both knees. 234 (30.5%) of these failed, 168 (22.9%) due to meniscal root tear, and 42 (5.4%) due to subchondral insufficiency fracture. There were 7 (0.9%) cases of findings suggestive of primary or metastatic tumor detected on MRI and 1 (0.1%) on knee X-ray. 30 (2.4%) people were excluded on hip and 4 (0.3%) on shoulder X-rays. 5 hip and 24 knee joints had arthroplasty, but these were not exclusionary. Several people exhibited more than one pathology, so reasons for exclusion slightly exceed the total number of people excluded.
Conclusion: A significant proportion of people with OA show radiologic findings at screening. Excluding these patients is important to distinguish existing pathologies from treatment-emergent events in early trials. Rigorous radiologic surveillance supported determination of LEVI-04 joint safety; LEVI-04 was not associated with an increase in adverse joint events compared to placebo in this study1. Phase 3 trials are in planning. Conaghan P, et al. Arthritis Rheumatol. 2024;76 (suppl 9).
To cite this abstract in AMA style:
Guermazi A, Conaghan P, Perkins C, Herholdt C, Bombelka I, Westbrook S. Radiologic surveillance in the Phase II RCT of LEVI-04, a novel neurotrophin-3 inhibitor, in people with knee osteoarthritis: exclusions at screening [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/radiologic-surveillance-in-the-phase-ii-rct-of-levi-04-a-novel-neurotrophin-3-inhibitor-in-people-with-knee-osteoarthritis-exclusions-at-screening/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/radiologic-surveillance-in-the-phase-ii-rct-of-levi-04-a-novel-neurotrophin-3-inhibitor-in-people-with-knee-osteoarthritis-exclusions-at-screening/