Session Information
Date: Saturday, November 7, 2020
Title: SLE – Animal Models Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Lupus Erythematosus (SLE) is an autoimmune disease with an incompletely understood etiology. Previous work has identified Rab4A, a small GTPase responsible for endosomal recycling, to be a potential pathogenic driver of disease [Caza, et al. Ann Rheum Dis. 2014], which involves activation of mTOR that is responsive to accumulation of kynurenine. Here, we investigated whether Rab4A activity controls kynurenine metabolism through regulating surface expression of CD98 via recycling.
Methods: Using the wild-type parental line Sle1.2.3. (Sle Rab4aWT), constitutively active (Sle Rab4AQ72L), or T-cell deleted Rab4A (Sle Rab4ACD4-KO) were generated using a Lox-Cre system. Splenocytes were harvested from the spleens of mice (n=12) and analyzed by flow cytometry using antibodies against CD3, CD4, CD8, CD19 and tryptophan/kynurenine transporter, CD98. Metabolomic data was acquired using a Q-Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer (ThermoFisher). CD4+ T cells were isolated using a positive selection kit (ThermoFisher), then stimulated for 72hrs with anti-CD3 and anti-CD28 antibodies. The metabolomic profile of Sle Rab4Q72L (n=4) and SLERab4CD4-KO (n=4) were compared using Metaboanalyst 3.0. Disease progression was assessed by glomerulonephritis (GN) scoring [1]. Statistical analysis was carried with ANOVA and Student’s t-test using GraphPad.
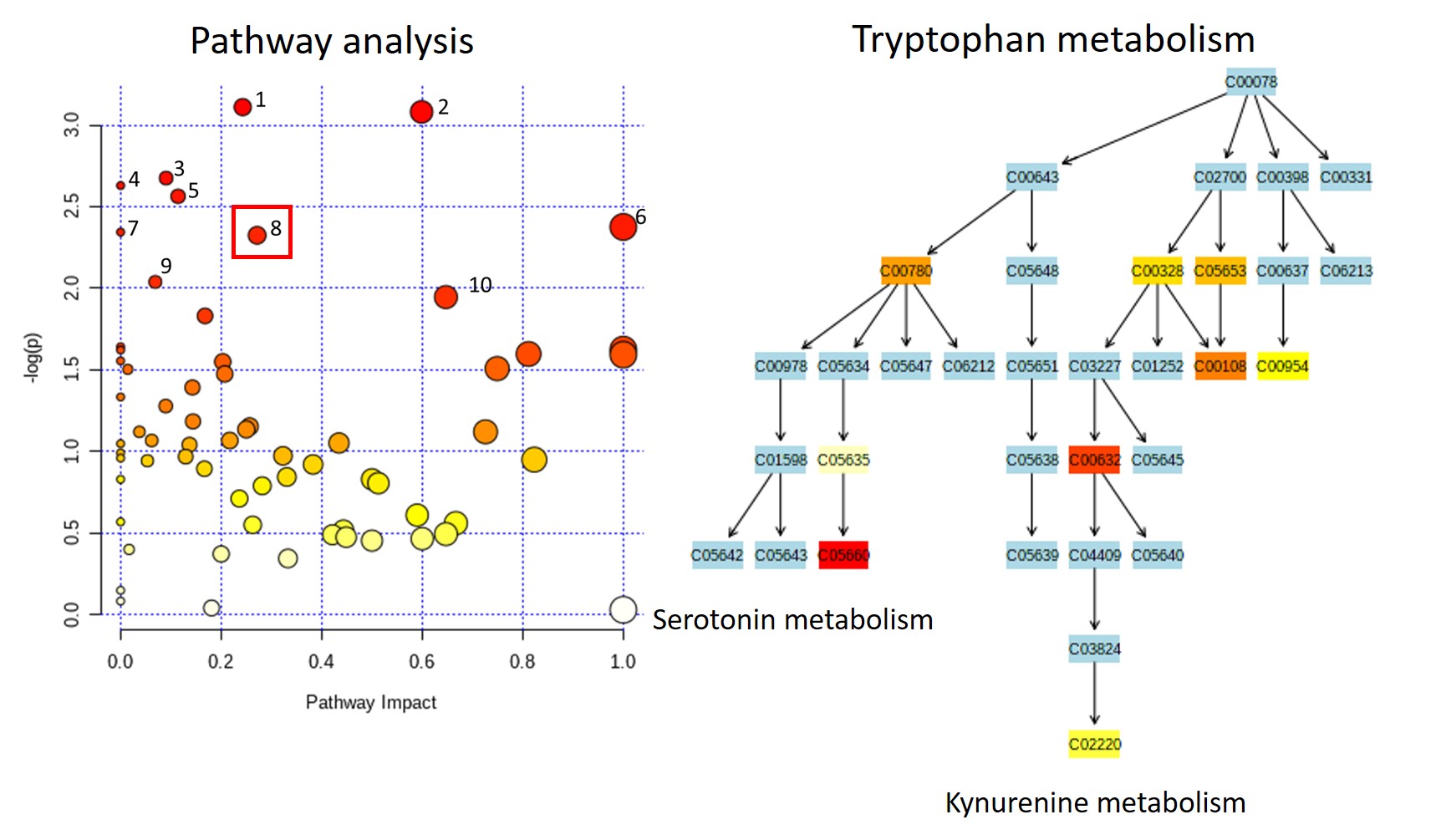
Results: Mean GN score was significantly reduced in Sle Rab4aCD4-KO (0.3) compared to Sle Rab4AWT (1.1, p=0.039) and Sle Rab4AQ72L mice (1.5, p=0.001). In pathogenic CD3+CD4-CD8- double-negative (DN) T cells, CD98 expression was significantly increased in Sle Rab4AQ72L compared to Sle Rab4aWT (p=0.029) and Sle Rab4ACD4-KO mice (p=0.019) (Figure 2). This trend was also seen in CD4+ T cells without reaching significance (Figure 2). CD98hi cells were depleted in Sle Rab4ACD4-KO compared to Sle Rab4AWT (p=0.044) and Sle Rab4AQ72L mice (p=0.040). The tryptophan metabolomic pathway was measured by mass spectrometry to determine downstream effects of CD98 recycling by Rab4A. In Sle Rab4AQ72L mice, accumulation of several tryptophan metabolites, including kynurenine, anthranilate, and serotonin was seen compared to Sle Rab4ACD4-KO mice (Figure 3). A global impact on tryptophan metabolism was supported by pathway analysis effectively discriminating between Sle Rab4AQ72L and Sle Rab4ACD4-KO mice.
Conclusion: Rab4A activity controls the onset and severity of GN Sle1.2.3. lupus-prone mice. Constitutively active Rab4A has increased glomerulonephritis, while deleting Rab4A in T cells ameliorates disease activity. Activation of Rab4A promotes the expression the CD98 and the accumulation of kynurenine and other tryptophan metabolites, suggesting that Rab4A-dependent tryptophan and kynurenine uptake may control mTOR activation seen in human subjects with SLE.
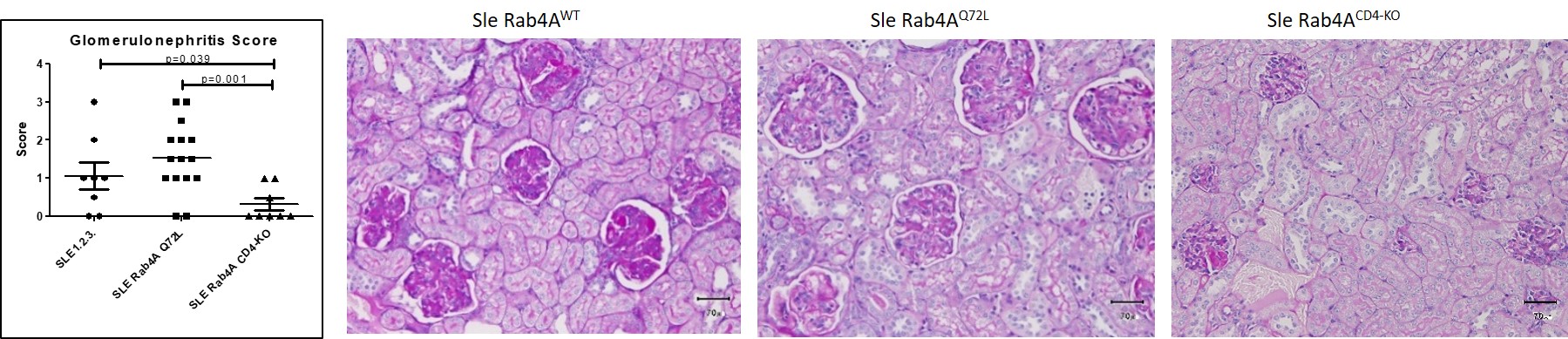 Figure 1. Glomerulonephritis scoring. Kidney sections from Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice were scored blind on a 0-4 scale. Left, scores of n=31 mice. Right, representative sections in Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice.
Figure 1. Glomerulonephritis scoring. Kidney sections from Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice were scored blind on a 0-4 scale. Left, scores of n=31 mice. Right, representative sections in Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice.
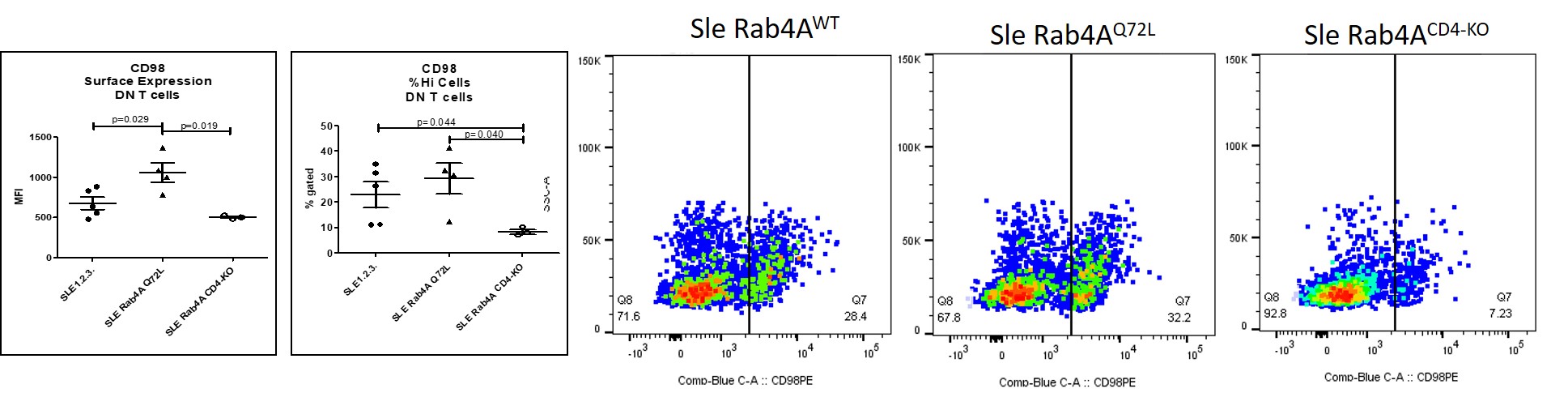 Figure 2. CD98 surface expression in CD4+ T cells and DN T cells. Surface expression was measured by flow cytometry. Left, mean fluorescent intensity and prevalence of CD98hi cells. Right, representative dotplots showing CD98 expression in DN T cells in Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice.
Figure 2. CD98 surface expression in CD4+ T cells and DN T cells. Surface expression was measured by flow cytometry. Left, mean fluorescent intensity and prevalence of CD98hi cells. Right, representative dotplots showing CD98 expression in DN T cells in Sle Rab4AWT, Sle Rab4AQ72L, and Sle Rab4ACD4-KO mice.
To cite this abstract in AMA style:
Wyman B, Huang N, Lai Z, Haas M, Duarte M, Lewis J, Perl A. Rab4A Regulates Glomerulonephritis and Tryptophan Metabolism in Sle1.2.3. Lupus-prone Mice via Recycling of CD98 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/rab4a-regulates-glomerulonephritis-and-tryptophan-metabolism-in-sle1-2-3-lupus-prone-mice-via-recycling-of-cd98/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rab4a-regulates-glomerulonephritis-and-tryptophan-metabolism-in-sle1-2-3-lupus-prone-mice-via-recycling-of-cd98/