Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: HRES-1/Rab4 (Rab4A) is a small GTPase that is overexpressed in SLE patient T cells1,2, mediates the enhanced recycling of CD3 and CD4 cell surface receptors1,2, and the activation of the mechanistic target of rapamycin (mTOR)3. Recently, increased expression of CD384, mTOR activation5, and loss of IL-2 production6,7 have been implicated in pro-inflammatory T cell development in SLE. In this study, we investigated the impact of Rab4A on the expression of CD38 and the secretion of IL-2 and characterized the impact of CD38 expression on the activation of mTOR in CD4+ T cells.
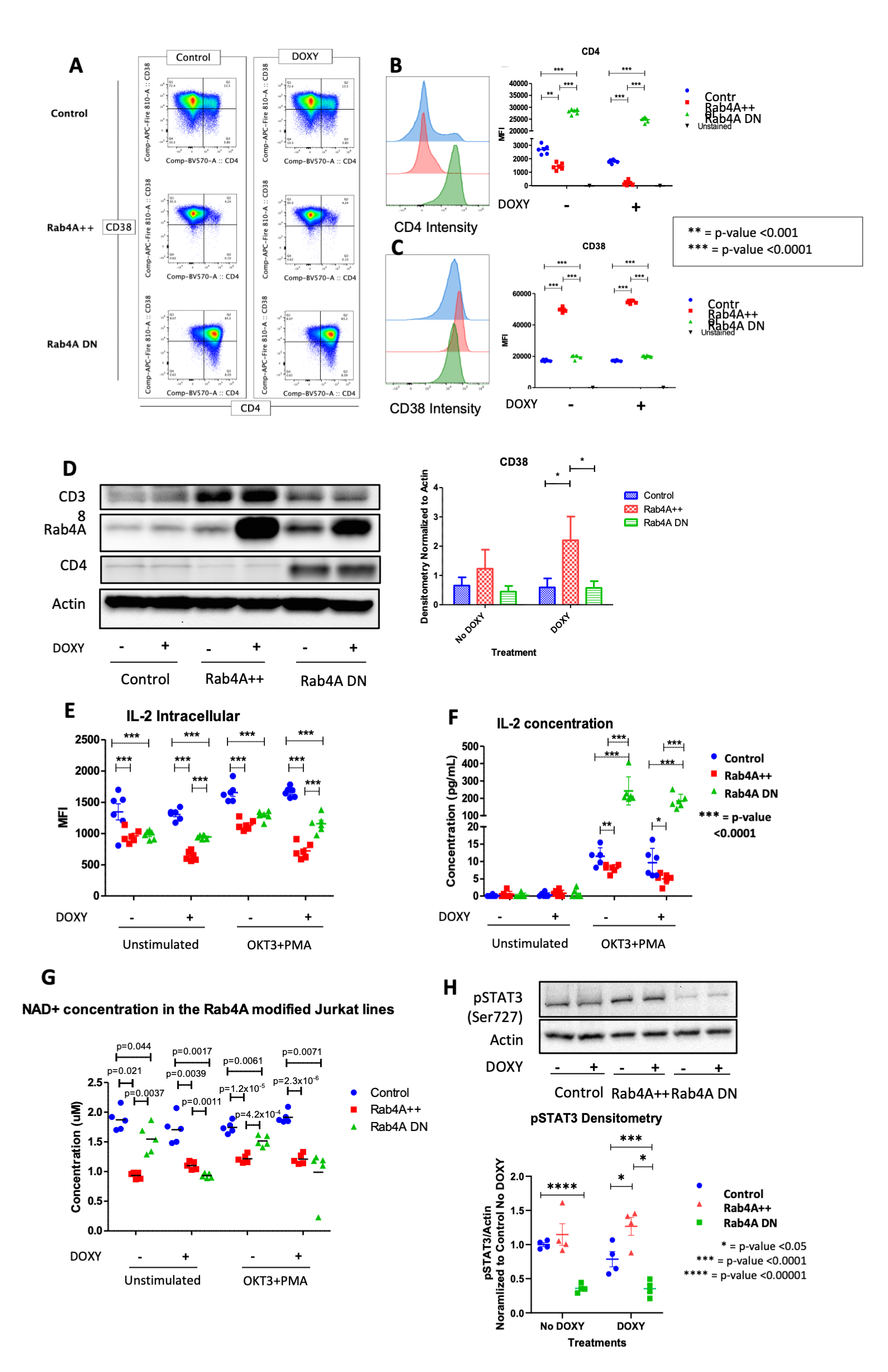
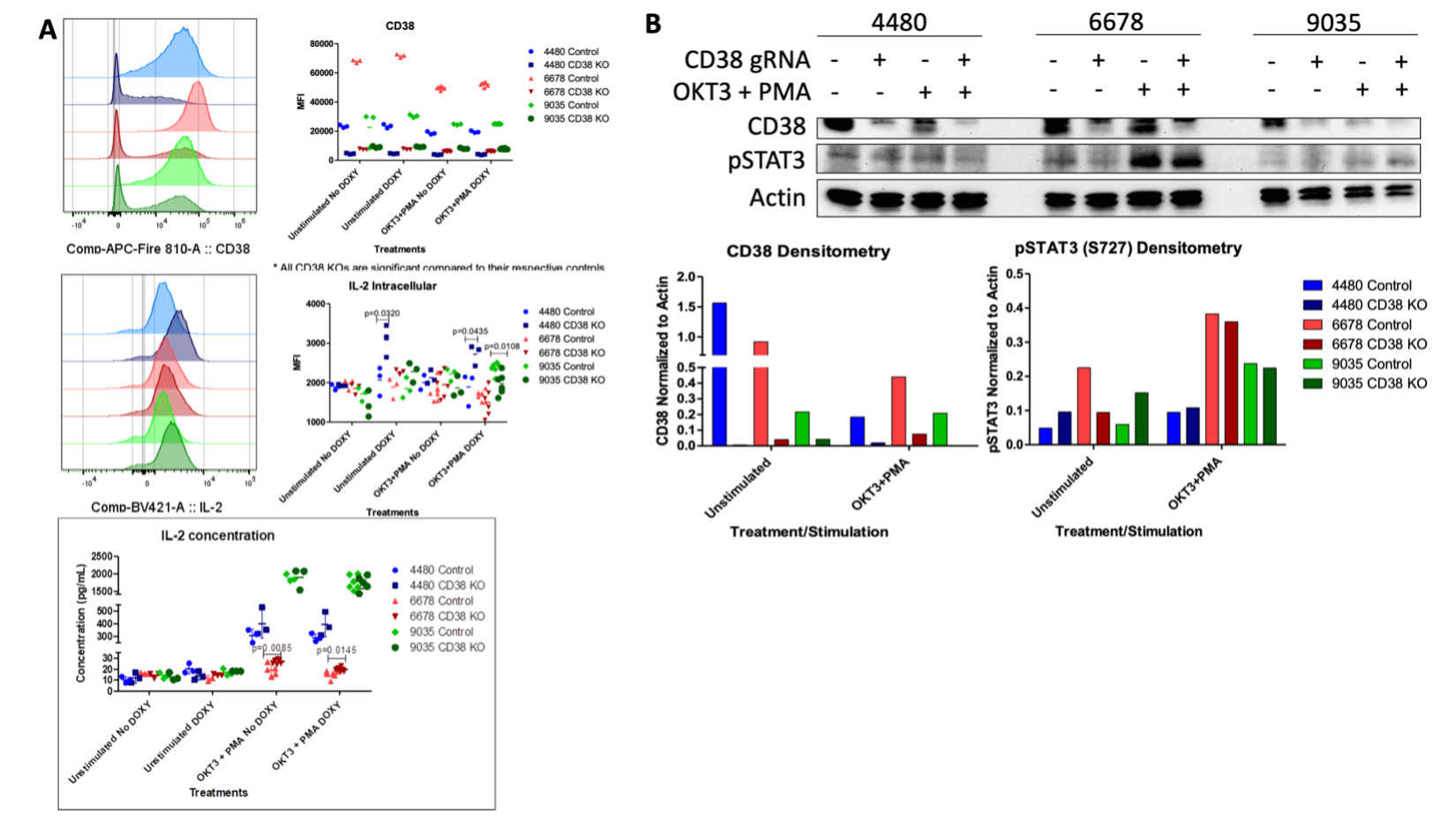
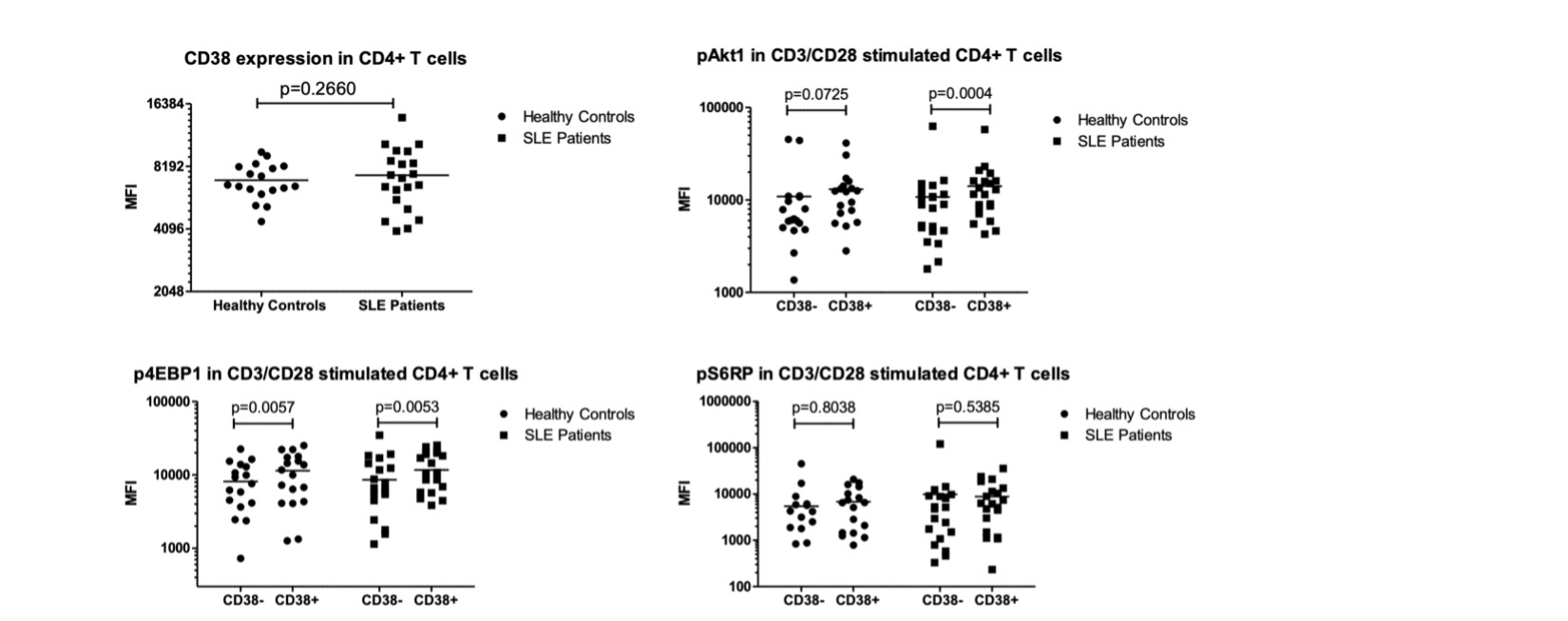
Methods: To understand the cellular consequences of Rab4A overexpression, our lab has created unique Rab4A-mutant Jurkat cell lines, which contain GFP-expressing vector alone (control), doxycycline-inducible vectors that overexpress Rab4A (Rab4A++) or the dominant-negative mutant Rab4AS27N (Rab4ADN)8. We also CRISPR knocked out (KO) CD38 in these cell lines, leading to six different lines: (1) Rab4AWT CD38WT, (2) Rab4AWT CD38KO, (3) Rab4A++ CD38WT, (4) Rab4A++ CD38KO, (5) Rab4ADN CD38WT, and (6) Rab4ADN CD38KO. These cells were cultured with doxycycline and co-stimulated with anti-CD3 mAb (OKT3) and phorbol myristate acetate (PMA) to induce cytokine production9. Cell surface markers and cytokines were analyzed by flow cytometry and protein levels by western blot. NAD+ levels were measured by LC-MS/MS. To understand the impact of CD38 expression on mTOR activation, we isolated peripheral blood mononuclear cells from SLE patients (n=21) and age, sex, and race matched healthy controls (n=18). The cells were cultured for 24 hours with and without CD3/CD28 co-stimulation and were analyzed by flow cytometry.
Results: In the Rab4A++ cells compared to the control, CD38 expression was upregulated (p=2.49×10-13), intracellular production and secretion of IL-2 significantly decreased (p=1.16×10-7 and p=0.0401, respectively), NAD+ concentration decreased (p=0.0039), while pSTAT3 levels increased (p=0.0318). In the Rab4A++ CD38KO cells compared to the Rab4A++ CD38WT cells, secretion of IL-2 significantly increased (p=0.0145) and pSTAT3 levels decreased. pAkt1 was increased significantly in CD38+ CD4+ T cells compared to CD38– CD4+ T cells in only the SLE patients (p=0.0004), while p4EBP1 increased significantly in CD38+ CD4+ T cells compared to CD38– CD4+ T cells for both the SLE patients (p=0.0053) and healthy controls (p=0.0057).
Conclusion: The increased pAkt1 and p4EBP1 in SLE patients’ CD38+ CD4+ T cells suggests that CD38 activates mTOR via Akt, coinciding with a recent finding of CD38/PI3K/Akt/mTOR axis in cervical cancer10. CD38 is an NAD+ hydrolase, which regulates Sirtuin-1 activity, a NAD+-dependent histone deacetylase that suppresses STAT3 activity. STAT3 activation is also known to be regulated by mTOR11–13. Elevated pSTAT3 levels may underlie diminished IL-2 production by binding to the promoter of FoxO1, which inhibits IL-2 production. The overexpression of Rab4A, increased CD38, pAkt1, p4EBP1, and pSTAT3, and diminished IL-2 production reflect changes observed in SLE patients. Our results suggest that increased expression of Rab4A and CD38 may underlie the diminished secretion of IL-2 in SLE.
To cite this abstract in AMA style:
Park J, Wang X, Godavarthy A, Patel A, Daniel K, Nolan J, Chilton J, Blaker B, Perl A. Rab4A Controls the Depletion of IL-2 in CD4+ T Cells via Enhanced CD38 Expression: Potential Involvement in Proinflammatory Lineage Development in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/rab4a-controls-the-depletion-of-il-2-in-cd4-t-cells-via-enhanced-cd38-expression-potential-involvement-in-proinflammatory-lineage-development-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rab4a-controls-the-depletion-of-il-2-in-cd4-t-cells-via-enhanced-cd38-expression-potential-involvement-in-proinflammatory-lineage-development-in-systemic-lupus-erythematosus/