Session Information
Date: Sunday, October 26, 2025
Title: (0671–0710) Systemic Sclerosis & Related Disorders – Clinical Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with early severe diffuse cutaneous systemic sclerosis (dcSSc) with interstitial lung disease (ILD) have poor prognosis. New tools are needed to improve treatment stratification and monitoring. Studies with repeated positron emission tomography-computed tomography with [18F]Fluorodeoxyglucose ([18F]FDG PET-CT) in this specific high-risk group have not been performed and may have clinical value. In this study we aimed to (1) assess changes in [18F]FDG lung uptake between baseline and follow-up after different treatment regimens (2) identify if [18F]FDG lung uptake at baseline is associated with ILD progression during follow-up.
Methods: [18F]FDG PET-CT scans of the lungs were performed at baseline and repeated one year after initial randomization to cyclophosphamide pulse therapy or autologous stem cell transplantation within the UPSIDE trial (NCT04464434). All [18F]FDG PET-CT scans were analyzed by manual placement of 2 cm volumes of interest (VOIs) in 22 pre-defined lung areas and mean standardized uptake values (SUVs) of [18F]FDG tracer uptake were corrected for the uptake in the mediastinal blood pool. Percentual changes after one year were calculated by the mean of all VOIs. Changes in [18F]FDG lung uptake were compared to clinical assessment of ILD development. At 1 year follow-up, ILD progression was defined according to the Progressive Pulmonary Fibrosis (PPF) criteria (2 out of 3 criteria): (1) increased respiratory symptoms, (2) decreased FVC≥5% or DLCO≥10% (3) increased lung fibrosis on HRCT, while patients met criteria for ILD improvement with (2 out of 3) reversed criteria: (1) decreased respiratory symptoms, (2) increased FVC≥5% or DLCO≥10% (3) decreased lung fibrosis on HRCT. Stable ILD was defined as having neither progression nor improvement. Between group differences were analyzed using non-parametric tests.
Results: Eleven patients were included. Five patients were female, with a median age of 54 years and median disease duration of 9.5 months at baseline. Patients with ILD progression at 1 year (n=3) showed increased [18F]FDG uptake (corrected SUVmean +21.7%) after 1 year, while patients with improved ILD (n=4) showed decreased [18F]FDG uptake (corrected SUVmean -19.4%) over time (Figure 1 and 2A). Less evident differences were observed in patients with stable ILD (n=4; corrected SUVmean -1.5%). Finally, [18F]FDG uptake at baseline did not differ between patients who did or did not progress at 1 year of follow-up (Figure 2B).
Conclusion: [18F]FDG uptake in the lungs changed in line with clinical response to therapy in patients with early severe dcSSc and ILD. These results suggests that [18F]FDG PET-CT has the potential to have additive value in the monitoring of SSc-ILD.
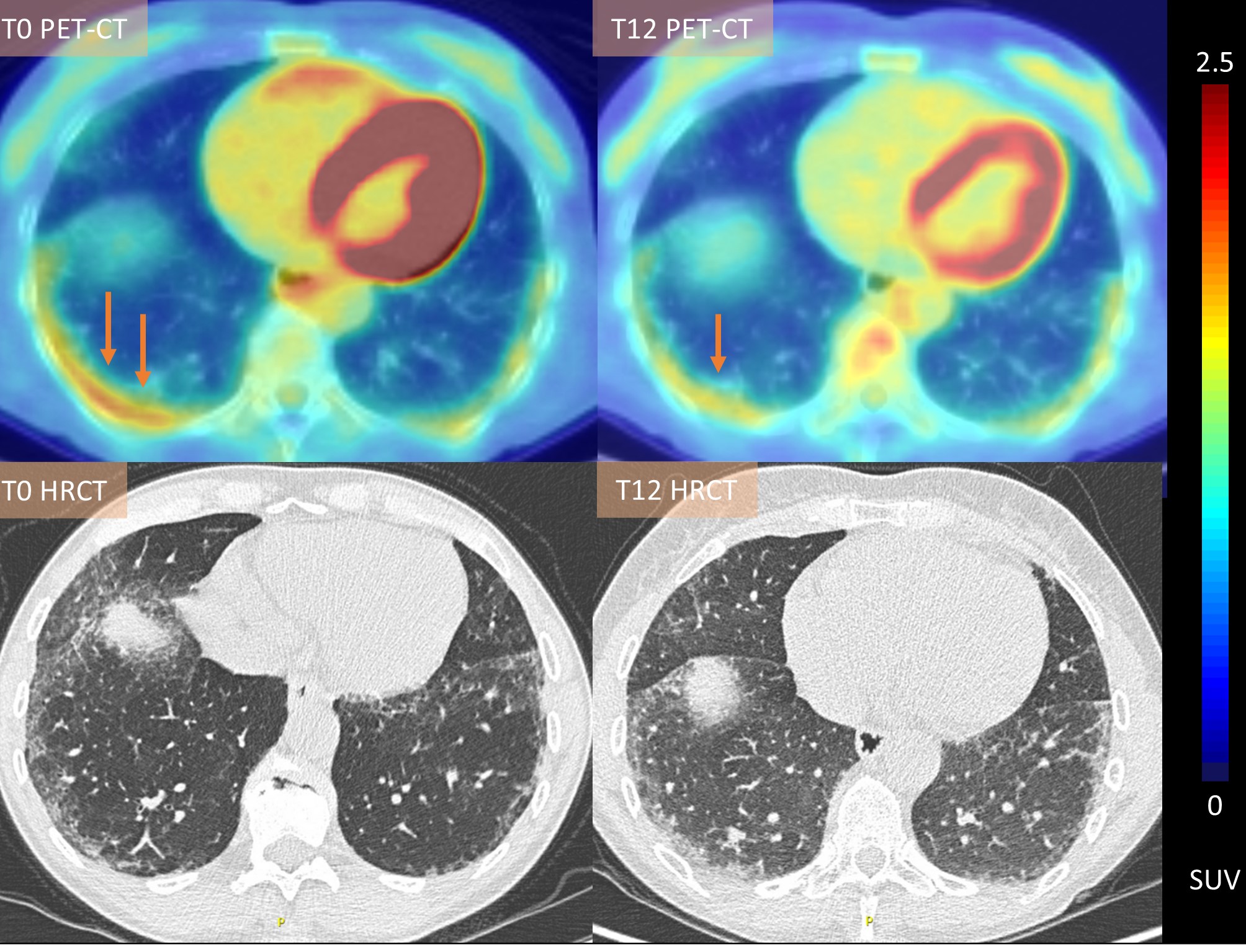 Figure 1. Visual and quantitative decrease in pulmonary [18F]FDG uptake in a patient with early severe diffuse cutaneous systemic sclerosis and interstitial lung disease with clinical improvement
Figure 1. Visual and quantitative decrease in pulmonary [18F]FDG uptake in a patient with early severe diffuse cutaneous systemic sclerosis and interstitial lung disease with clinical improvement
.jpg) Figure 2. (Changes in) Quantitative [18F]FDG uptake between different patient groups (improved, stable or progressed interstitial lung disease)
Figure 2. (Changes in) Quantitative [18F]FDG uptake between different patient groups (improved, stable or progressed interstitial lung disease)
To cite this abstract in AMA style:
Broens B, Nossent e, Meijboom L, Zwezerijnen G, Spierings j, de Vries-Bouwstra J, van Laar J, van der Laken C, Voskuijl A. Quantitative 18F-FDG PET-CT of the lungs detects treatment induced changes in patients with early severe diffuse cutaneous systemic sclerosis and interstitial lung disease [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/quantitative-18f-fdg-pet-ct-of-the-lungs-detects-treatment-induced-changes-in-patients-with-early-severe-diffuse-cutaneous-systemic-sclerosis-and-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantitative-18f-fdg-pet-ct-of-the-lungs-detects-treatment-induced-changes-in-patients-with-early-severe-diffuse-cutaneous-systemic-sclerosis-and-interstitial-lung-disease/
