Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
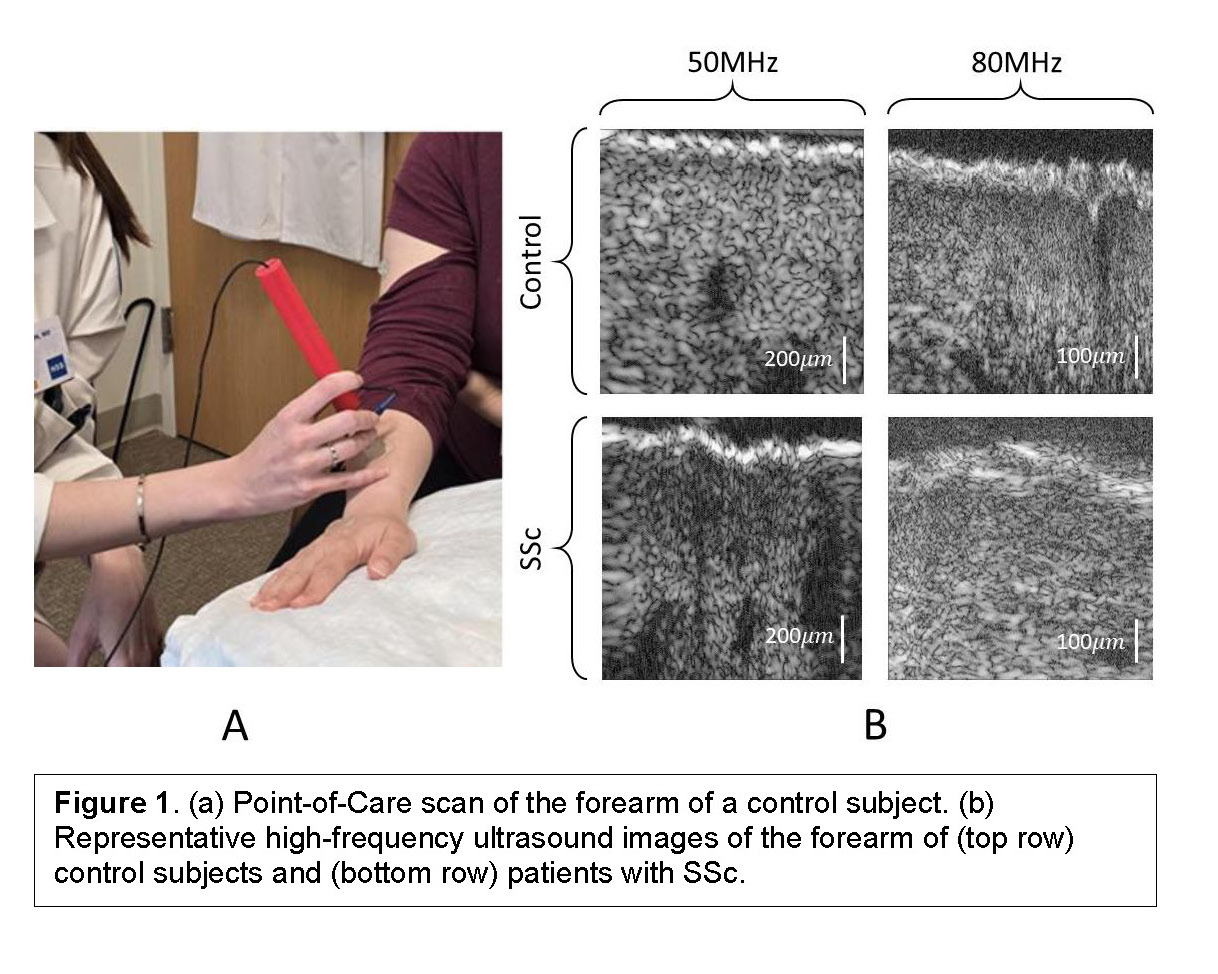
Background/Purpose: A highly reproducible, quantitative method of measuring skin severity in systemic sclerosis (SSc) trials and patient care is needed. While conventional skin US, operating in the 1-10 MHz range, can measure SSc skin thickness, high-frequency, quantitative ultrasound (QUS) presents a unique opportunity to non-invasively assess other key microarchitecture features including edema, fibrosis, and microstructural changes in collagen. We have developed a novel point-of-care (POC) high-frequency instrument built to the specifications of SSc skin by retrospectively analyzing >50 SSc skin biopsies. The POC instrument is equipped with two handheld probes operating at 50-MHz (Fig. 1a) and 80-MHz, respectively. Unlike conventional US devices, the high frequencies of the POC instrument yields QUS parameters sensitive to tissue structures 6-10 µm in diameter, providing quantitative insight into the microstructure of the collagen in SSc skin. The purpose of this pilot in-vivo study is to compare QUS parameters between SSc and healthy skin using the novel POC SSc-QUS device.
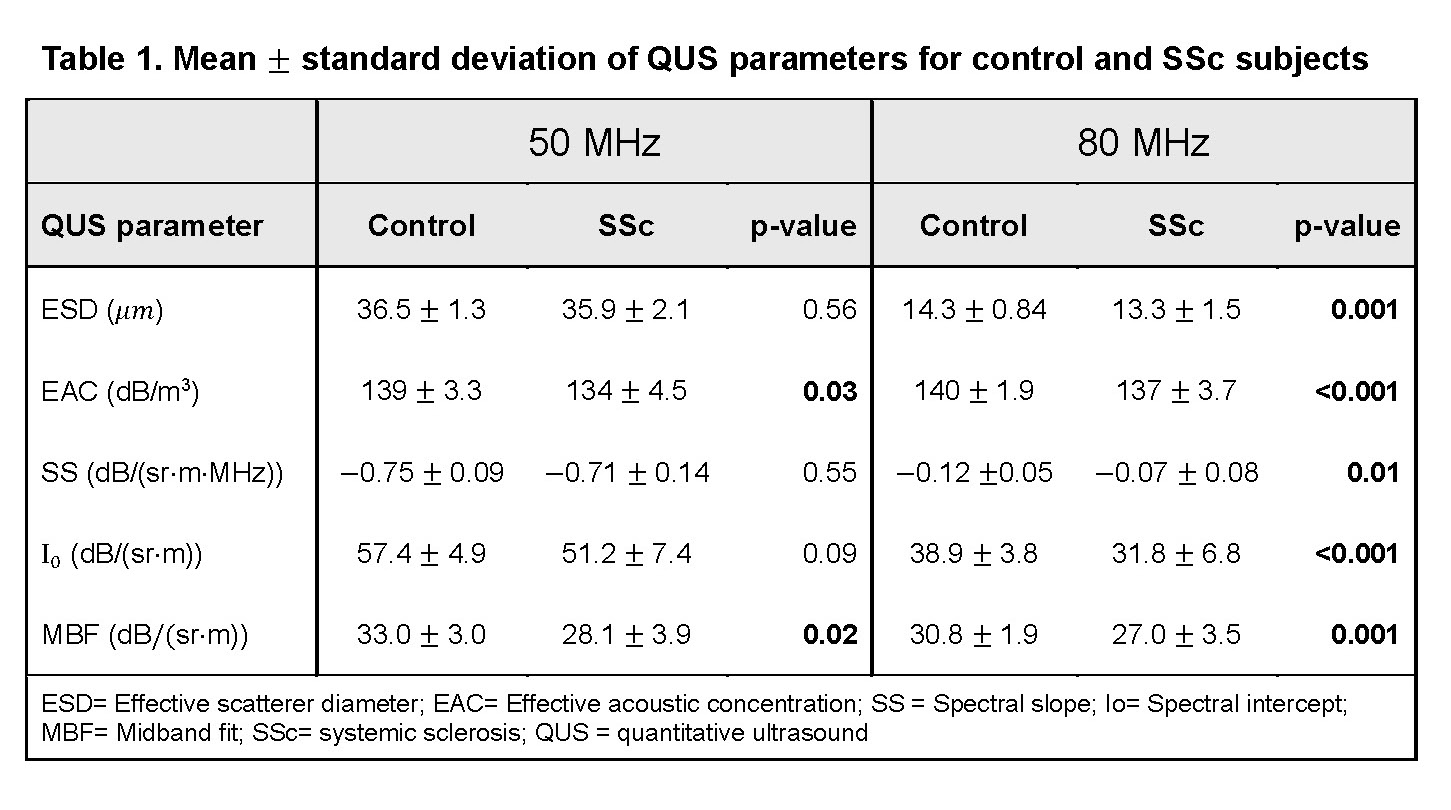
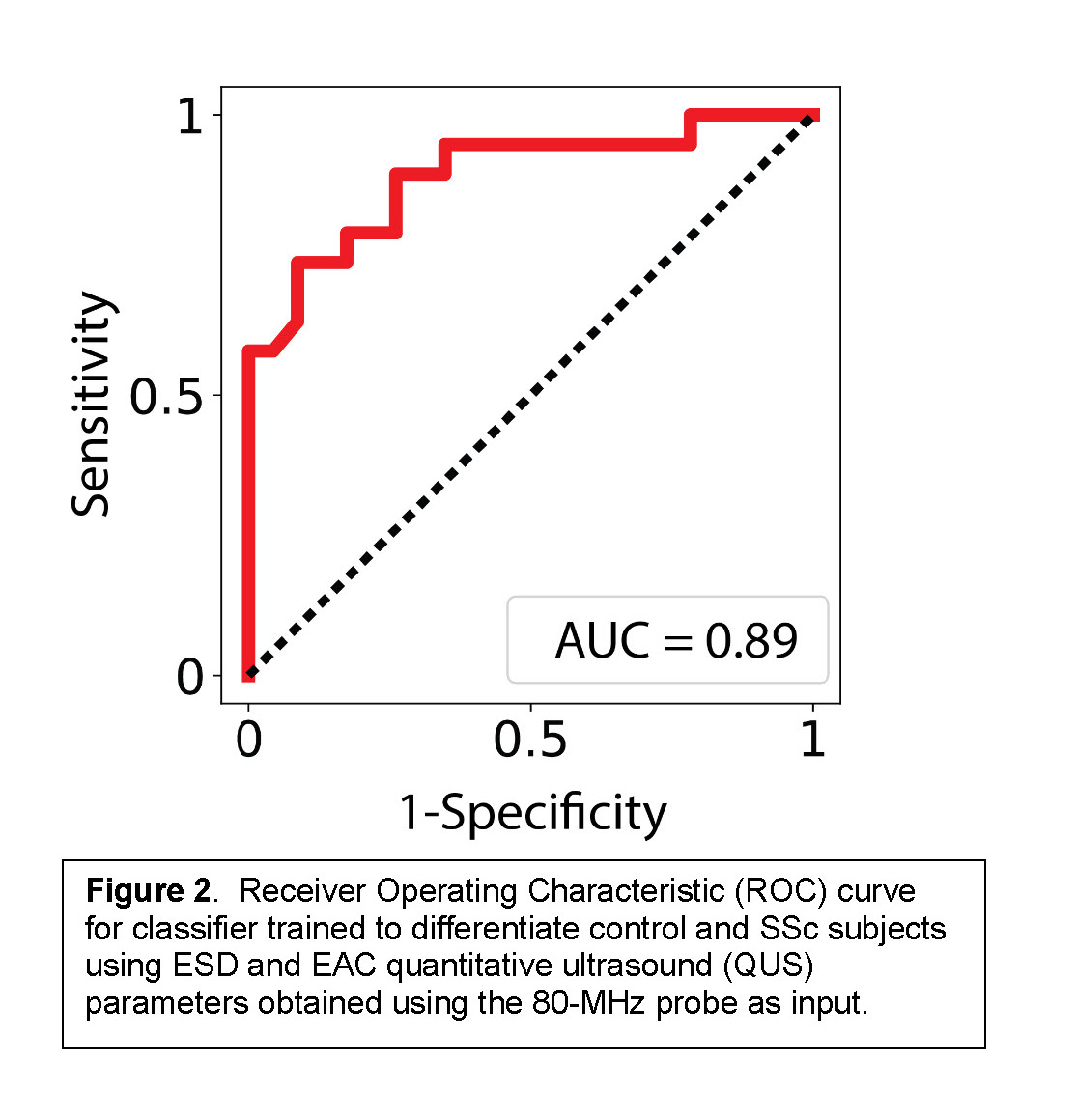
Methods: Healthy controls without inflammatory skin disease (N=8) and individuals with diffuse SSc (N=6) were recruited for POC-based high-frequency QUS (50, 80MHz) assessments of both forearms. Five backscatter coefficient-based QUS parameters, effective scatterer diameter (ESD), effective acoustic concentration (EAC), spectral slope (SS), spectral intercept (I0), and midband fit (MBF), that relate to tissue microstructure, were compared between SSc patients and controls using an independent-samples t-test. A support vector machine (SVM) classifier was trained using a radial basis function kernel and ESD and EAC as input features. Classifier performance was evaluated using a receiver-operating characteristic curve (ROC) and calculating the area under the ROC (AUC).
Results: Median (IQR) age of controls and SSc patients were 30 (25, 33) and 54 (48, 59), respectively. Median (IQR) total modified Rodnan Skin score (mRSS) was 24 (17, 34), local (US-site) mRSS was 2 (1, 3), and disease duration was 3.2 (1.4, 5.3) years. Representative high-frequency ultrasound images from the forearm of controls and SSc patients are shown in Fig. 1b. Significant differences in all five QUS parameters were noted between controls and SSc patients at 80 MHz (very high frequency), but only in EAC and MBF at 50 MHz (Table 1). A classifier trained using ESD and EAC computed from data acquired using the 80-MHz probe as inputs was able to differentiate SSc and control subjects with a sensitivity, specificity, and AUC of 90%, 74%, 0.89, respectively (Fig. 2).
Conclusion: These pilot data demonstrate that high frequency (80MHz) QUS parameters clearly distinguish between SSc and healthy skin. Lower frequency (50MHz) parameters, EAC and MBF, marginally distinguished between healthy controls and SSc patients in this small sample, suggesting that the 80MHz device is highly sensitive to SSc-related microstructural changes. Longitudinal studies are underway to correlate QUS parameters with SSc skin severity and trajectory in a larger sample of patients and controls to test QUS as a useful quantitative outcome measure for SSc clinical trials and patient care.
To cite this abstract in AMA style:
Lakin K, Hoerig C, Gordon J, Ghahramani E, Diaz M, Spiera R, Orange d, Mamou J. Quantifying Tissue Microstructural Changes in Scleroderma Skin Using a Novel, Point-of-care, High-frequency-ultrasound Instrument [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/quantifying-tissue-microstructural-changes-in-scleroderma-skin-using-a-novel-point-of-care-high-frequency-ultrasound-instrument/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantifying-tissue-microstructural-changes-in-scleroderma-skin-using-a-novel-point-of-care-high-frequency-ultrasound-instrument/