Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Corticosteroids (CS) are commonly used for rheumatologic conditions, and known to cause systemic adverse events (AEs), particularly when used at high doses for prolonged periods. The burden, the incidence of AEs, and associated costs were assessed across varying levels of CS exposure.
Methods: Patients with a diagnosis of select autoimmune and inflammatory diseases between 1/1/2006 and 9/30/2015 were selected from a de-identified claims database. Patients with ≥60 days of continuous CS use were stratified into three prednisone-equivalent dose cohorts: ≤7.5 mg/day ("low dose"), >7.5-≤15 mg/day ("medium dose"), or >15 mg/day ("high dose"). These groups were compared with patients with <60 days of CS use. Outcomes were assessed from the first day of a patient’s highest dose episode until the earliest of 30 days after the end of continuous use, plan disenrollment, or end of study period. The incidence of AEs and costs during follow up were compared across cohorts.
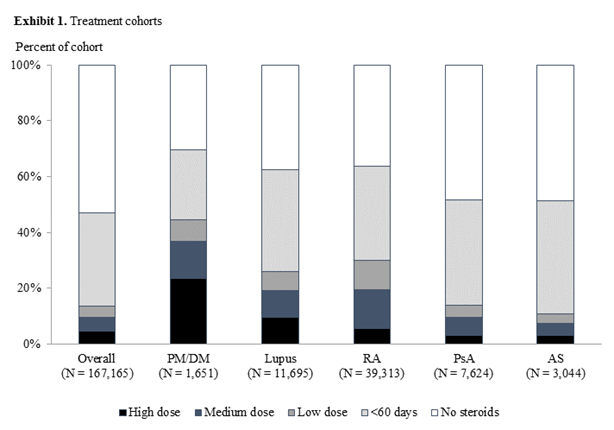
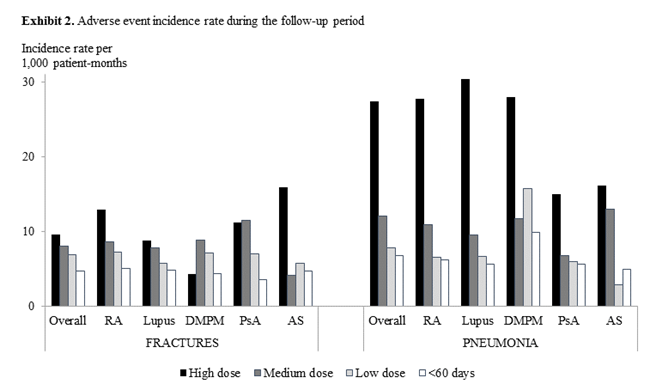
Results: Among the 167,165 included patients, one-third had <60 days of CS use, 4% had low, 5% had medium, and 4% had high dose use of ≥60 days. High and medium-dose CS use varied among the conditions: PM/DM (37%), lupus (19%), and RA (19%) (Exhibit 1). Compared to patients in the <60 days cohort, risks of AEs (new cases) were several folds higher in the high-dose cohort: myocardial infarction (incidence rate ratio [IRR] 3.8), pneumonia (IRR 4.1), glaucoma (IRR 2.3), and hypertension (IRR 2.4). Most AEs had increasing incidence with higher-dose use (Examples shown in Exhibit 2). Across the three ≥60-day cohorts, AEs occurred at an average of 2.3-6.7 months after the initiation of the CS episodes; AEs developed more quickly for patients with high-dose than medium- or low-dose CS use. All treatment cohorts had higher AE-related medical costs than disease-related medical costs (Exhibit 3). AE-related medical costs accounted for approximately 30% of the mean annualized total healthcare costs (27% for <60 day patients, 33% for high-dose patients).
Conclusion: This study quantified and demonstrated a dose-relationship for the risks of and time to AEs as well as the costs of prolonged CS use. Among patients who are exposed to high-doses, considerations to reduce their reliance on CS may provide clinical and economic benefits.
|
Exhibit 3. Annualized patient healthcare costs |
||||
|
|
High dose |
Medium dose |
Low dose |
<60 days |
|
Total all-cause healthcare costs (including medical and prescription drug costs) |
$68,408 |
$35,498 |
$31,900 |
$32,690 |
|
Adverse event-related medical costs |
$22,807 |
$10,632 |
$9,342 |
$8,947 |
|
Disease-related medical costs |
$16,151 |
$6,846 |
$5,399 |
$4,133 |
|
Disease-related prescription drug costs |
$2,066 |
$3,491 |
$3,749 |
$1,507 |
|
Non-adverse event or disease-related healthcare costs |
$59,434 |
$27,160 |
$23,910 |
$28,132 |
To cite this abstract in AMA style:
Rice JB, White A, Lopez A, Wagh A, Qin Y, Mitri G, Bartels-Peculis L, Ciepielewska G, Nelson W. Quantifying Clinical and Economic Outcomes Associated with Chronic Corticosteroid Exposure in a US Population [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/quantifying-clinical-and-economic-outcomes-associated-with-chronic-corticosteroid-exposure-in-a-us-population/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantifying-clinical-and-economic-outcomes-associated-with-chronic-corticosteroid-exposure-in-a-us-population/