Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are susceptible for pneumococcal disease. Two vaccines against S. pneumoniae are currently recommended for use: a 23-valent polysaccharide vaccine (PPSV23) and a 13-valent pneumococcal CRM197 conjugate vaccine (PCV13). Stepwise pneumococcal vaccination, a PCV13 prime-PPSV23 boost strategy with an interval of at least 8 weeks between the two vaccinations, is now recommended by the Center for Disease Control and Prevention (CDC). Yet, real-life evidence shows a suboptimal uptake of pneumococcal vaccination among AIIRD patients.
The primary purpose of the project was to increase pneumococcal vaccine uptake, implementing the PCV13 prime-PPSV23 boost strategy among the adult AIIRD population within an Israeli nationwide health organization. The secondary aim was to investigate potential causes for non-adherence with the vaccination plan.
Methods: Quality improvement (QI) intervention program conducted within Maccabi Health Services (MHS) during 2019-2020 included electronic reminders linked order sets and outreach to patients with adult AIIRD identified by ICD-9-CM and MHS codes along with educational activities. We investigated the pneumococcal vaccination rate following the QI program and identified the factors associated with vaccination using univariate and multivariate logistic regressions. Multivariate models were adjusted for gender, age, COPD, CHF, prior influenza vaccination, and prior PPSV23 vaccination.
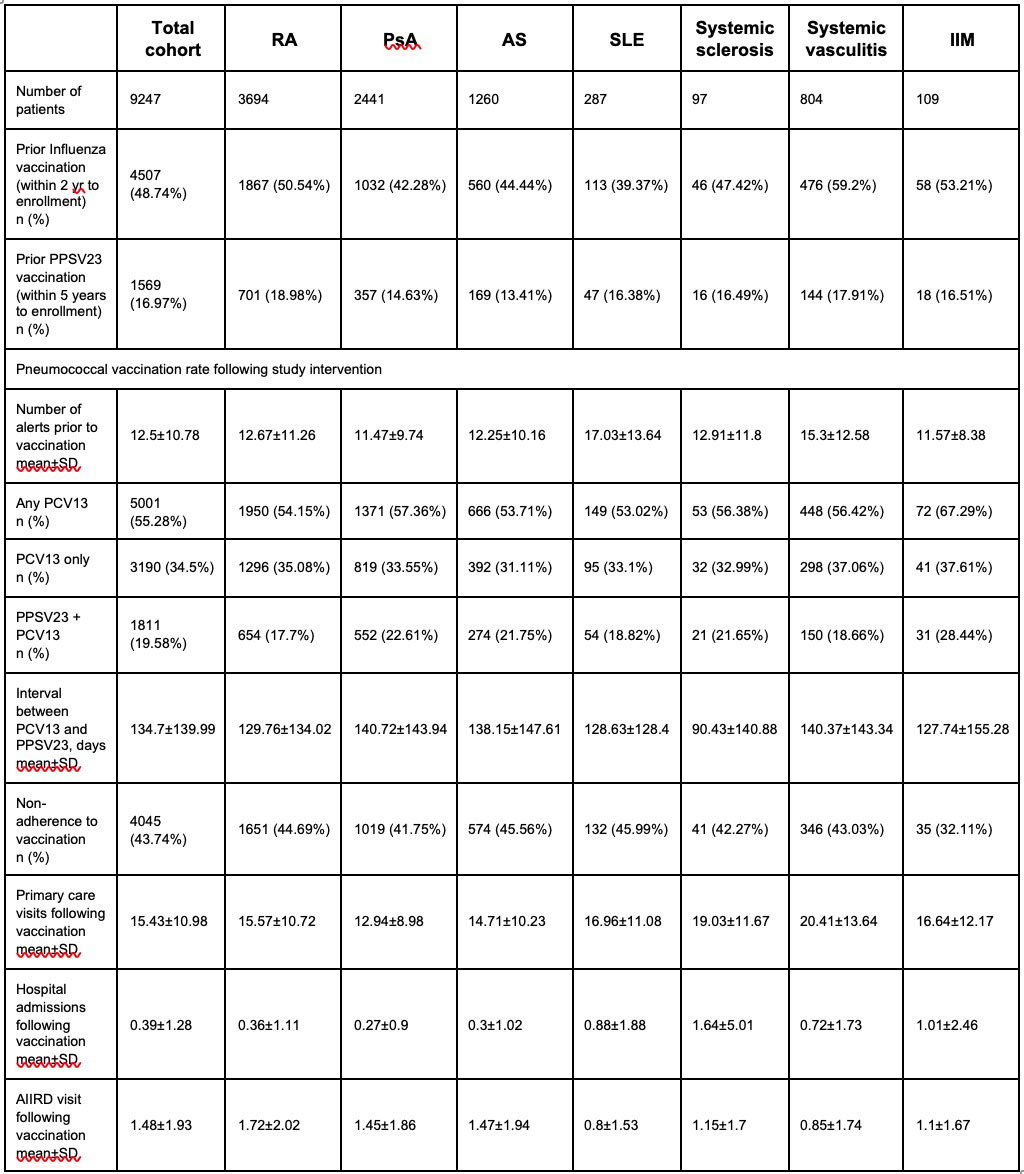
Results: A total of 9,247 AIIRD patients (62.6% females, age (mean±SD) 58.5±16.7 years) were defined as candidates for pneumococcal vaccination. Rheumatoid arthritis, psoriatic arthritis (PsA), ankylosing spondylitis, and systemic vasculitis were the commonest diseases, 39.9% (n=3,694), 26.4% (n=2,441), 13.6% (n=1,260), and 8.7% (n=804), respectively (Table 1).
The QI program issued 12.5±10.8/patient electronic reminders within the electronic medical records for pneumococcal vaccination. Following the intervention, PCV13-PPSV23 and PCV13 vaccination rates were 19.6% (n=1,811) and 34.5% (n=3,190), respectively (Table 2). Patients with inflammatory myopathies (iIM) and PsA had the highest rates of the prime-boost vaccination, 28.4% (n=31) and 22.6% (n=552), respectively. A total of 43.7% (n=4,045) of patients did not respond to the study intervention.
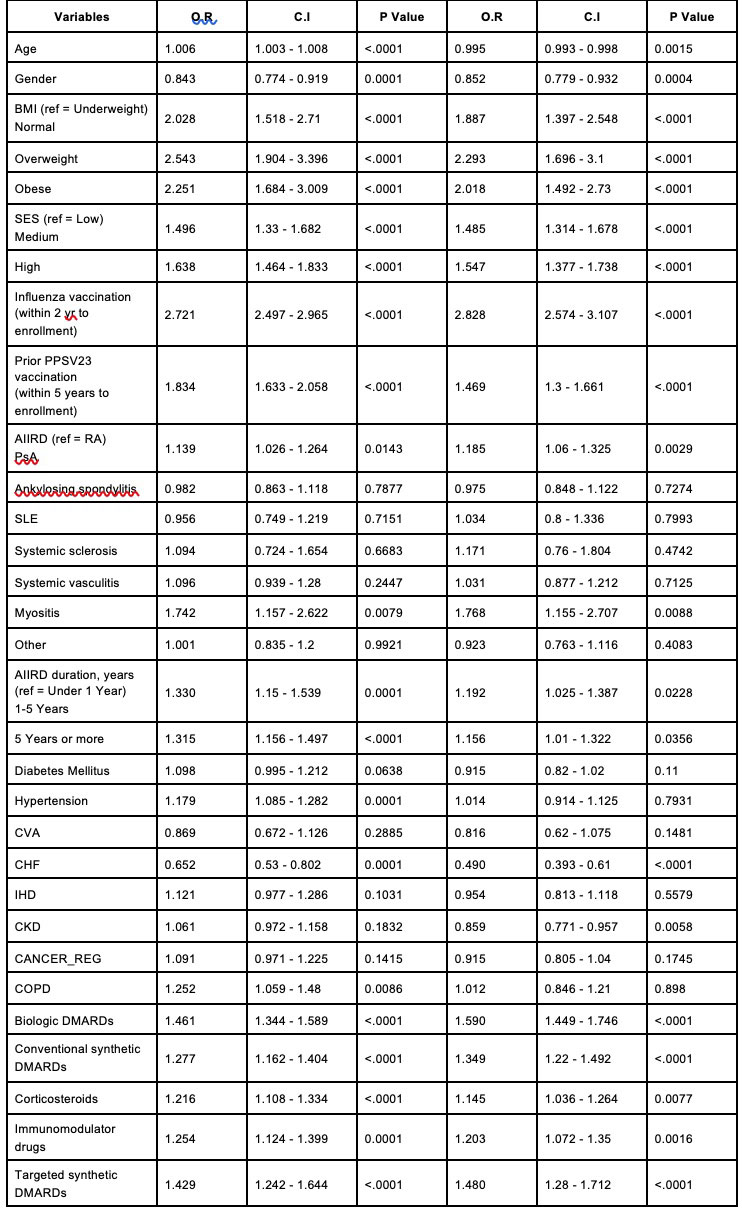
Adjusted predictors for the adherence with vaccination included overweight – OR 2.3, 95% CI 1.397-2.548, high socioeconomic status – OR 1.56, 95% CI 1.377-1.738, IIM – OR 1.768, 95% CI 1.155-2.707, biologic DMARDs – OR 1.590 95% CI 1.449-1.746, csDMARDs – OR 1.35 95% CI 1.220-1.492, immunosuppressants – OR 1.2 95% CI 1.072-1.350, tsDMARDs – OR 1.48 95% CI 1.28-1.71, and CS – OR 1.145 95% CI 1.036-1.264 (Table 3). Utilization of medical services, including primary care visits and rheumatology clinic visits, was higher in vaccinated compared to non-vaccinated AIIRD patients.
Conclusion: QI intervention program targeting the AIIRD population within a nationwide healthcare organization moderately increased the rate of pneumococcal vaccination. There remains a significant scope to improve pneumococcal vaccination uptake among patients with AIIRD.
Multivariate models are adjusted to age, gender, COPD, CHF, Influenza vaccination, and prior PPSV23 vaccination
To cite this abstract in AMA style:
Furer V, Sarbagil-Maman H, Elkayam O. Quality Improvement Project to Increase the Rate of Vaccination with Pneumococcal Vaccines (PCV13 prime-PPSV23 Boost Strategy) Among Adult Patients with Autoimmune Inflammatory Rheumatic Diseases (AIIRD) Within Nationwide Health Organization [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/quality-improvement-project-to-increase-the-rate-of-vaccination-with-pneumococcal-vaccines-pcv13-prime-ppsv23-boost-strategy-among-adult-patients-with-autoimmune-inflammatory-rheumatic-diseases-aii/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quality-improvement-project-to-increase-the-rate-of-vaccination-with-pneumococcal-vaccines-pcv13-prime-ppsv23-boost-strategy-among-adult-patients-with-autoimmune-inflammatory-rheumatic-diseases-aii/