Session Information
Session Type: Poster Session B
Session Time: 5:00PM-6:00PM
Background/Purpose: Children with Juvenile Idiopathic Arthritis (JIA) have better disease outcomes with current medications available, yet there is variability in these outcomes. Quality improvement (QI) processes are dynamic ways to identify gaps, implement goals and positively impact variability in outcomes. Organizations worldwide have identified QI goals for JIA that are important and feasible in their settings. Our practice opened in 2018, as the sole provider of pediatric rheumatology services in a small country. We identified QI goals that were published in the literature, essential for safety, and feasible in our setting with limited resources. We targeted four areas that posed safety risks: laboratory monitoring for DMARDs, tuberculosis screening prior to starting biologics, joint injection referrals, and uveitis screening. We describe the outcomes of our goals from 2018 to 2022.
Methods: From 2018 to 2022, we screened for drug toxicity among patients receiving methotrexate or leflunomide within 3-4 month of receiving methotrexate. We built measures that are captured and retrieved from the electronic medical record (EMR). From September 2021 to 2022, we added new QI goals that could not be captured by the EMR. We engaged dedicated nursing staff to monitor tuberculosis screening prior to starting biologics by ensuring completion of QuantiFERON gold or PPD testing, and/or chest x-ray. Our team developed a joint injection tracker to capture the number of patients having a procedure within 2 weeks of the referral. For uveitis screening and monitoring, we measured the percent of eligible JIA patients with up to date screenings over the previous 6 months. In January 2022, we implemented a monthly combined clinic where patients see Ophthalmology and Rheumatology together. Measures were reviewed quarterly.
Results: Between 2018 and 2022, laboratory monitoring for DMARDs showed that 100% of children receiving methotrexate or leflunomide, were screened for toxicity. However, during a COVID 19 surge, in the first quarter of 2021, 83% were screened. Between 2021 and 2022, 100% of patients had tuberculosis screening prior to starting biologics. Timely performance of joint injections was variable with a median of 53% done within 2 weeks. Prior to the combined clinics, 96% of patients had up to date eye screening visits. After the combined clinics, a median of 96.7% had up to date eye screening visits over 4 quarters.
Conclusion: QI projects can be successful and should start early by choosing and collecting the data needed to monitor improvement. Most of our goals were successful, except for the timeliness of procedures due to scheduling problems, patients canceling due to conflicts or illness. The EMR was able to capture one of our QI goals whereas other goals were not easily extractable from the EMR. Most of our QI measurements required the engagement of nursing staff to maintain the workflow with tracking sheets and reminders. The most successful goals were those that were completed by the rheumatology team at or before the visit. Finally, the implementation of a monthly, combined clinic was effective in having patients seen routinely and promptly. Sustainability is crucial and finding ways to automate cumbersome workflows are needed.
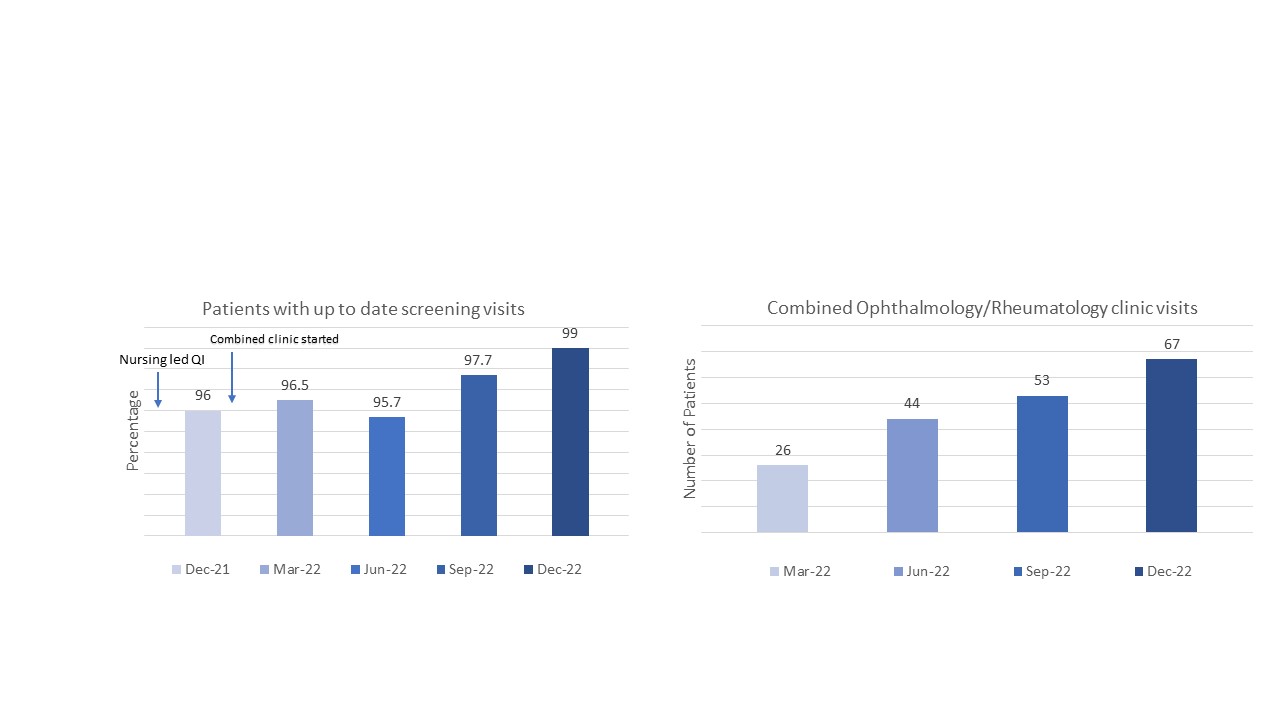 Patients with up to date eye visits improved after nursing engagement in the QI project and continued to improve after the implementation of combined clinics.
Patients with up to date eye visits improved after nursing engagement in the QI project and continued to improve after the implementation of combined clinics.
To cite this abstract in AMA style:
Shaya F, Bout-Tabaku S, Al-Adba B. Quality Improvement Lessons in a New Practice [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/quality-improvement-lessons-in-a-new-practice/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quality-improvement-lessons-in-a-new-practice/
