Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder that disproportionately impacts women of childbearing age. Although the incidence varies widely based on ethnic and geographic differences, approximately 204,000 persons in the US had SLE in 2018. To understand the nature and relative importance of skin symptoms, evaluate the content validity of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale in SLE, and assess what constitutes meaningful change in SLE-related fatigue and skin symptoms, this qualitative study held interviews with individuals with SLE.
Methods: In August 2023, individual interviews were conducted in the US in adults who reported receiving a diagnosis of SLE at least 6 months prior to screening and who experienced SLE-related fatigue, fever, joint pain, and/or skin rash/redness in the 30 days prior to their interview. Each 60-minute virtual interview was conducted in English in a semistructured format, using an interview guide to encourage response spontaneity and to ensure consistent, systematic data collection. Study participants described, in their own words, their experience of SLE-related fatigue and skin symptoms and what constituted minimal meaningful improvement in each symptom, using the Patient Global Impression of Severity (PGIS) and Patient Global Impression of Change (PGIC) scales. Cognitive debriefing and content validity of SLE-related fatigue were elicited using the 13-item FACIT-Fatigue scale. Regarding the ACR classification criteria for SLE, all diagnoses were self reported.
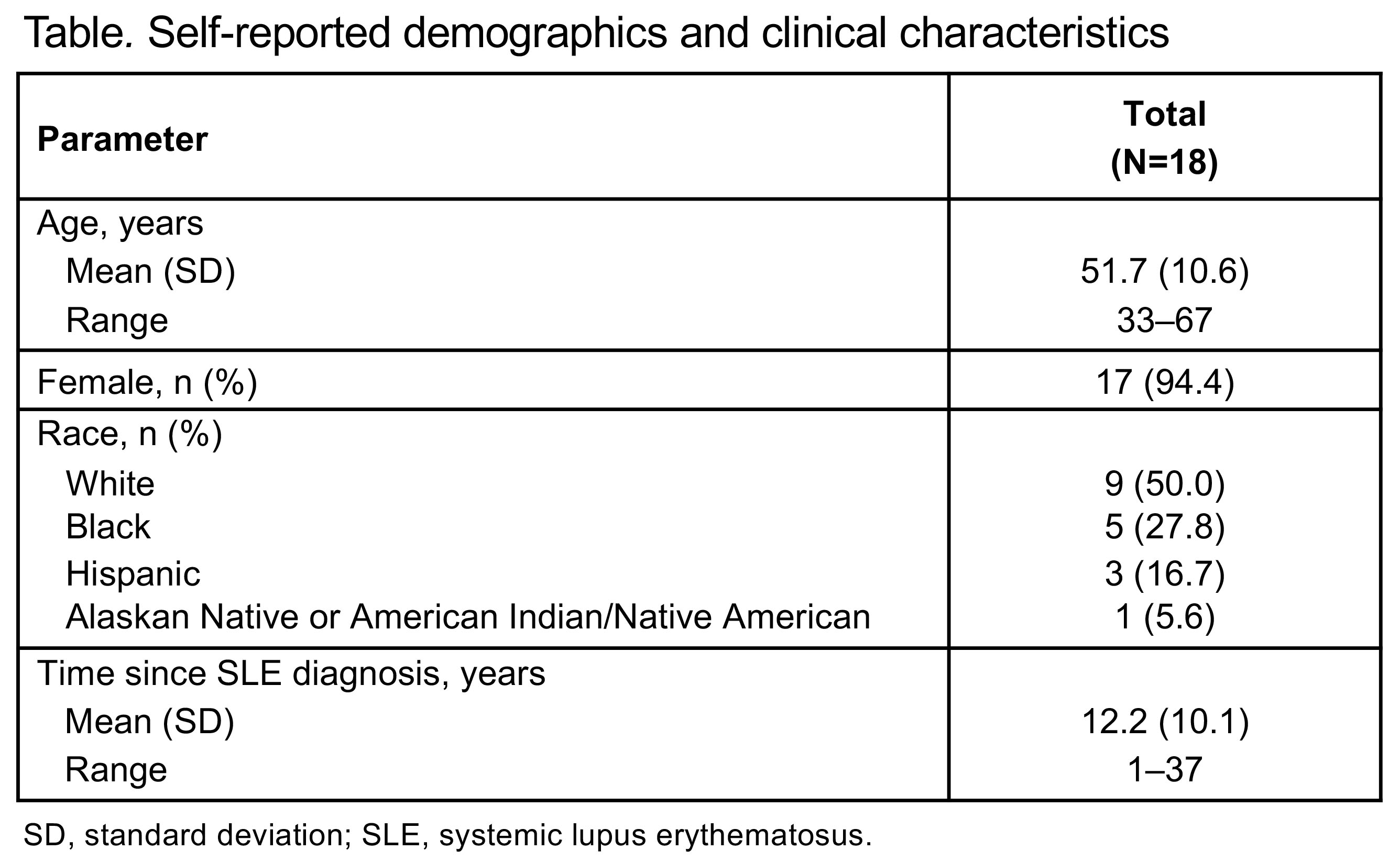
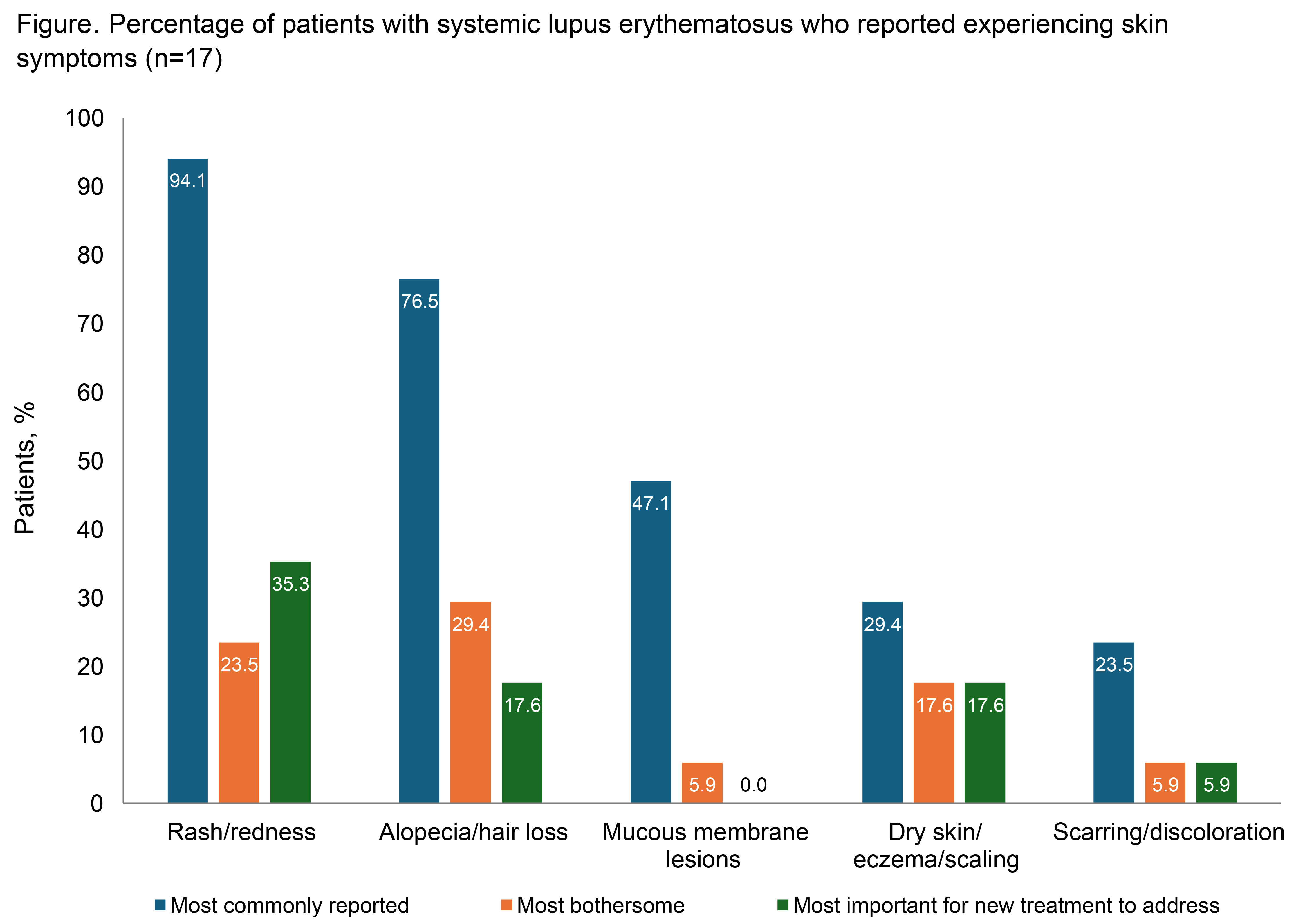
Results: The 18 participants interviewed had a mean age of 51.7 years (range, 33–67), 94.4% (n=17) were female, and 50.0% (n=9) were White (Table). Mean time since the clinical diagnosis of SLE was 12.2 years (range, 1–37). All but 1 participant reported experiencing SLE-related fatigue prior to or near the time of SLE diagnosis. This SLE-related fatigue was described as tiredness/exhaustion that sleeping does not help, lack of energy, feeling sluggish/heavy, and/or needing to rest/sleep during the day. Most participants (94.4%; n=17) were able to understand and answer all items without difficulty. The majority of participants (72.2%; n=13) also indicated that even a 1-point improvement in fatigue on the PGIS would be meaningful. Among participants reporting SLE-associated skin symptoms (n=17), rash/redness and alopecia/hair loss were the most frequently reported, most bothersome, and/or most important symptoms to be addressed by a new treatment (Figure). All participants understood the skin-specific PGIS and PGIC items without difficulty. Overall, 82.4% (n=14) indicated that a 1-point improvement on the PGIS would be meaningful, while 70.6% (n=12) indicated that “a little better” on the PGIC would represent meaningful improvement in skin symptoms.
Conclusion: These results support the content validity of the FACIT-Fatigue scale in SLE trials and suggest that even minimal improvement in fatigue and skin symptoms are meaningful to patients. New treatment options that can alleviate fatigue and skin symptoms are needed for patients with SLE.
To cite this abstract in AMA style:
Merola J, Werth V, Choi J, Becker B, Edwards T, Hobar C, Pomponi S, Strand V. Qualitative Patient Interview Study in Patients with Systemic Lupus Erythematosus to Assess Patient Perception of Fatigue and Skin-Related Symptoms [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/qualitative-patient-interview-study-in-patients-with-systemic-lupus-erythematosus-to-assess-patient-perception-of-fatigue-and-skin-related-symptoms/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/qualitative-patient-interview-study-in-patients-with-systemic-lupus-erythematosus-to-assess-patient-perception-of-fatigue-and-skin-related-symptoms/