Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Autoimmune disease patients treated with mycophenolate mofetil/mycophenolic acid (MMF/MPA), methotrexate (MTX), or B cell-depleting therapies (BCDT) exhibit reduced humoral responses following primary two-dose COVID-19 vaccination. However, the humoral response and clinical efficacy following a third (booster) vaccination are less studied, and prospective clinical trials analyzing the impact of withholding immunosuppression around the time of vaccination are limited. Here, we report the antibody responses, safety, and breakthrough infections for third vaccination in MMF/MPA-, MTX-, and BCDT-treated autoimmune disease patients and the effects of withholding MMF/MPA or MTX as part of the randomized, multi-site, open-label “Booster Effects with Autoimmune-Treatment in Patients with Poor Response to Initial COVID-19 Vaccine” trial (NCT#05000216).
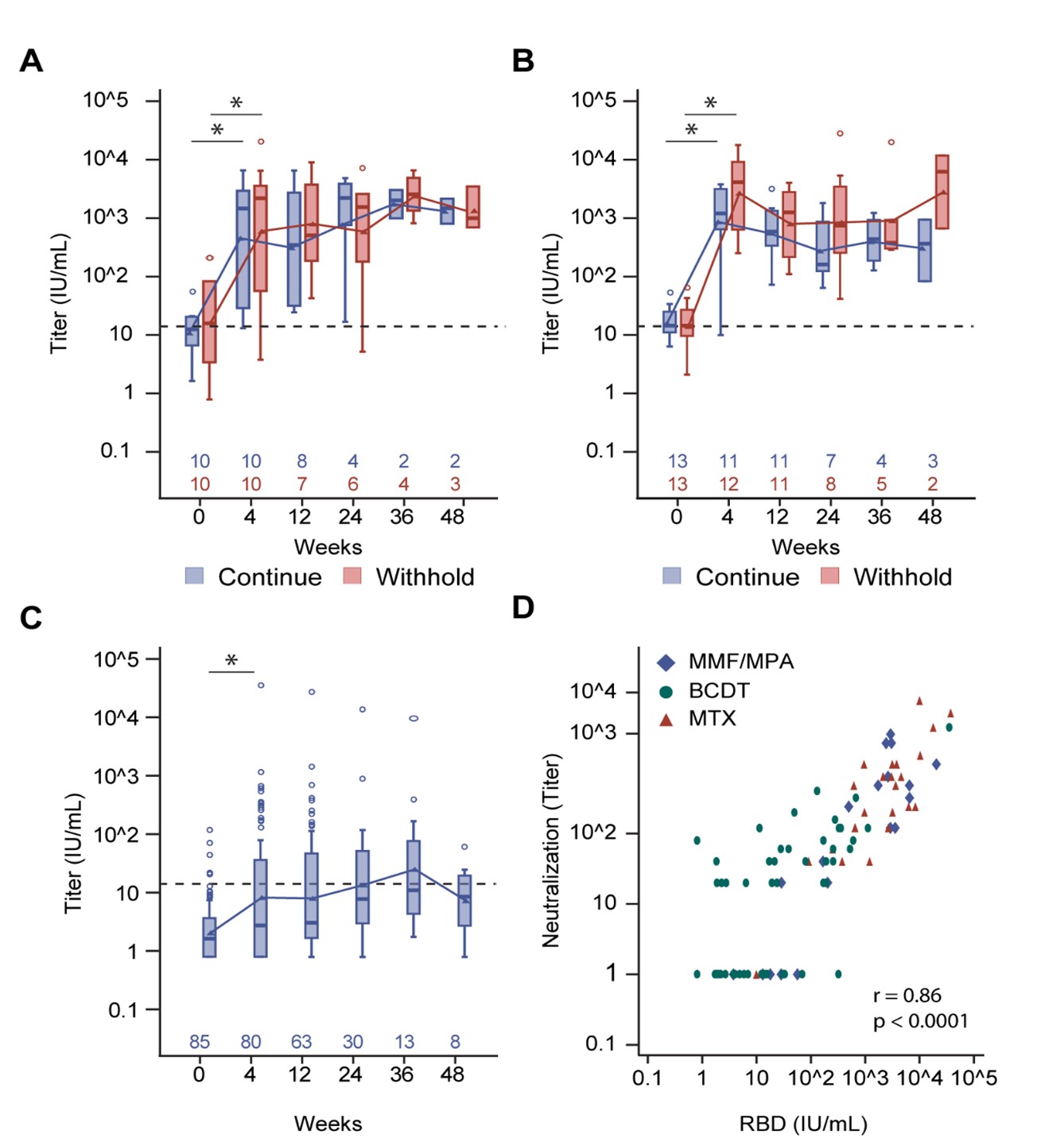
Methods: SLE, RA, SSc, multiple sclerosis, and pemphigus patients with negative or suboptimal (< 200 U/mL, Elecsys® Anti-SARS-CoV-2) antibody responses against the Wuhan-1 receptor-binding domain (RBD) following initial 2 doses of the SARS-CoV-2 BNT162b2 or mRNA-1273 vaccines received a homologous third vaccination at the baseline visit (MMF/MPA, n=20; MTX, n =26; BCDT, n=85). Of the MMF/MPA- and MTX-treated patients, 10 and 13, respectively, withheld treatment for 13-21 days around vaccination. Humoral responses against RBD were measured using the MSD 3-plex assay at baseline and 4, 12, 24, 36, and 48 weeks post-third vaccination. Neutralization was measured against the USA-WA1/2020 isolate.
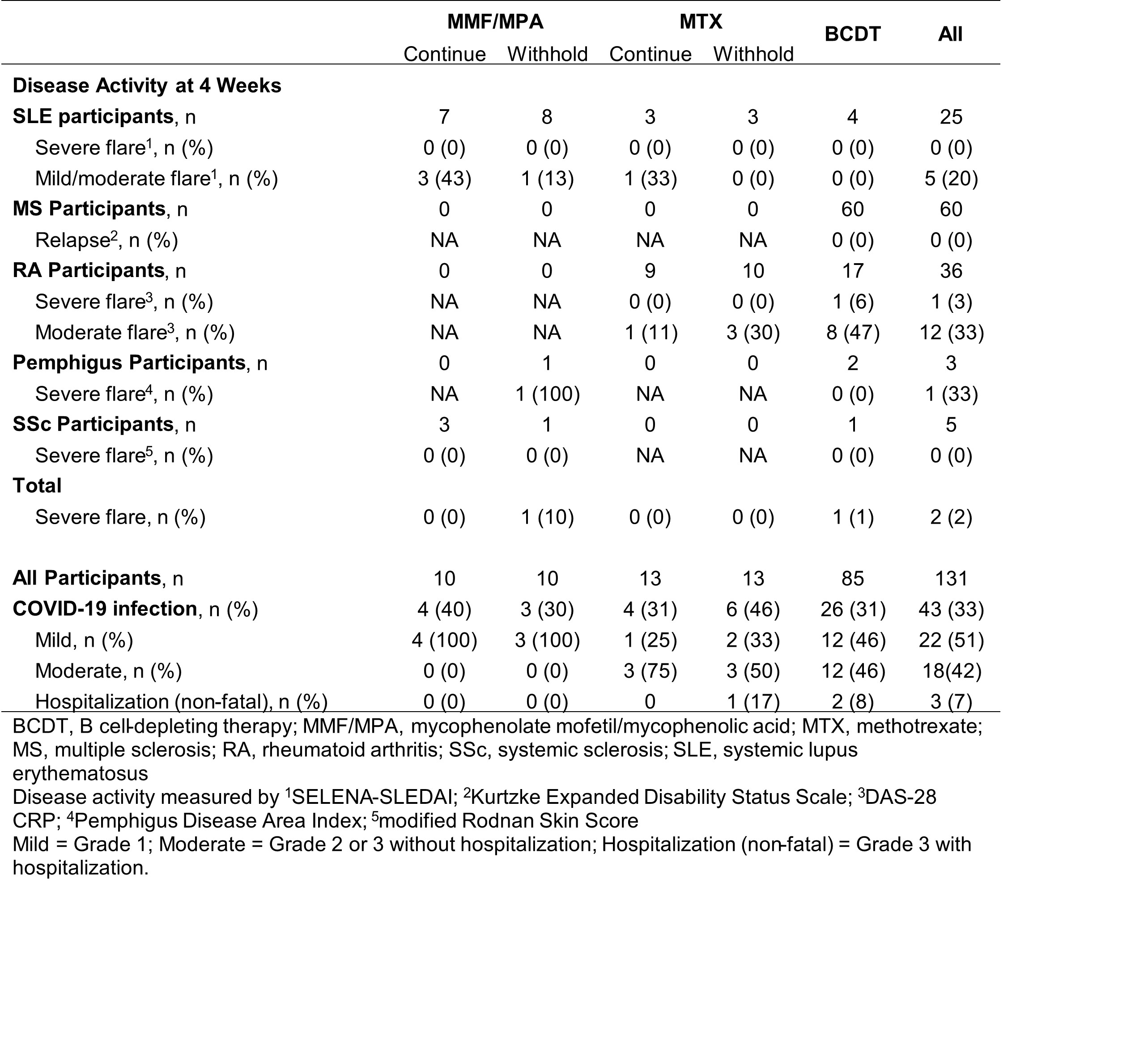
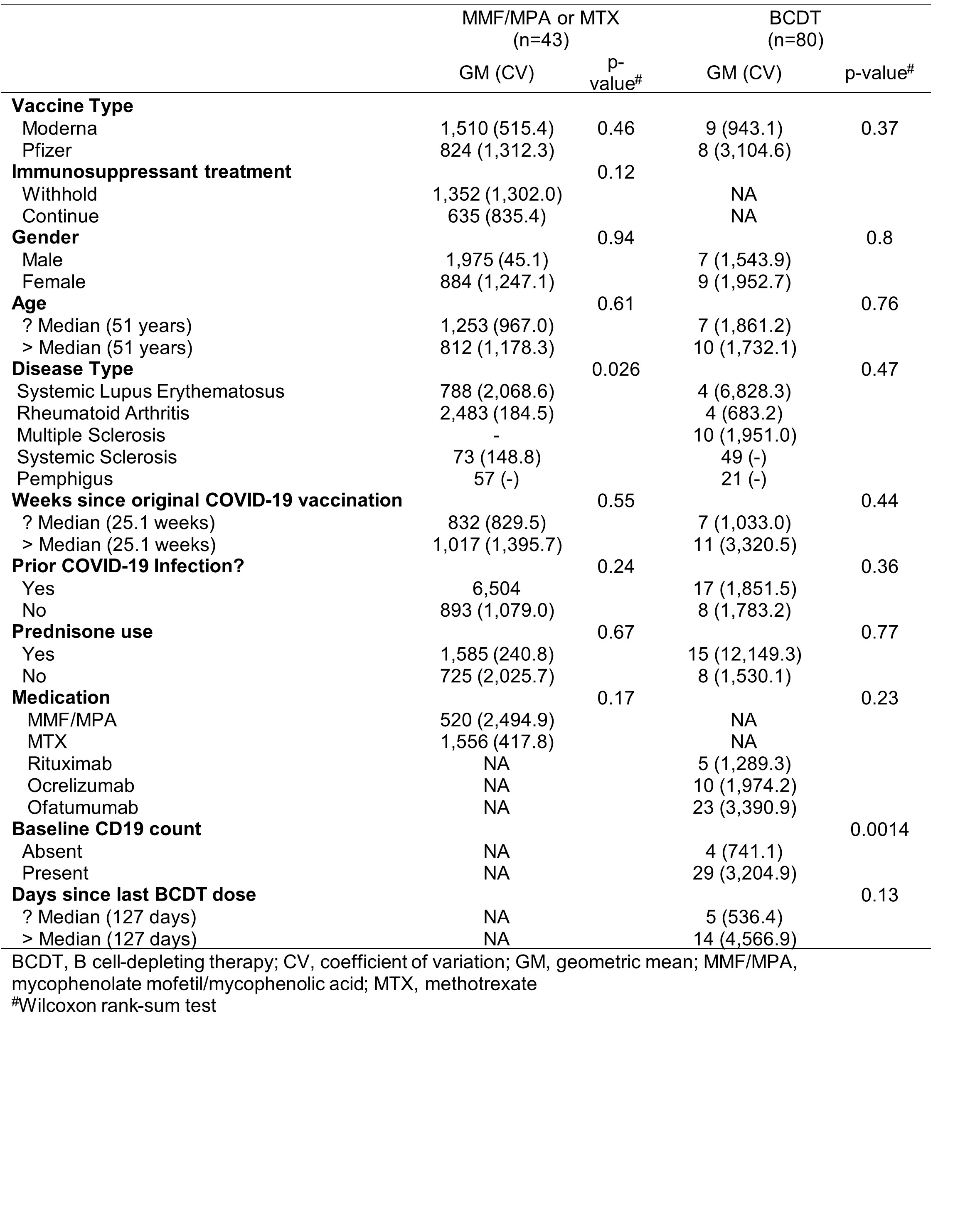
Results: In MMF/MPA- and MTX-treated autoimmune disease patients, a third vaccination increased anti-RBD seropositivity and concentrations at 4 weeks, regardless of immunosuppressive treatment withholding (Figure 1A-B). Although a third vaccination also increased anti-RBD responses in some BCDT-treated patients, only 36% were seropositive (Figure 1C). Anti-RBD responses at 4 weeks correlated with neutralization titers (r=0.86; p< 0.0001) (Figure 1 D). Humoral responses appeared sustained through 24 weeks in all groups, regardless of treatment withholding (Figure 1A-C). While post-vaccine disease flares occurred in 17 (13%) patients, severe flares were rare, occurring in only 1 RA (BCDT) and 1 pemphigus (MMF/MPA withheld) patient (Table 1). The frequency of breakthrough infections throughout the study ranged from 30-46%, with similar frequencies across treatment groups (Table 1). However, most breakthrough infections were mild, with only 3 non-fatal hospitalizations (Table 1). In BCDT-treated patients, detectable baseline B cell counts were associated with subsequent seropositivity against RBD (Table 2).
Conclusion: Third vaccination increases humoral responses to the SARS-CoV-2 RBD and does not impact disease activity across treatment groups regardless of immunosuppressive medication withholding. Although breakthrough infections occurred, they were typically mild in all treatment groups. Despite a limited sample size, our study supports booster vaccination in autoimmune disease patients receiving immunosuppressive medications.
NIAID/NIH Autoimmunity Centers of Excellence Award (U19AI110483; IS).
To cite this abstract in AMA style:
Mackay M, Wagner C, Pinckney A, Cohen J, Wallace Z, Khosroshahi A, Sparks J, Lord S, Saxena A, Caricchio R, Kim A, Kamen D, Koumpouras F, Askanase A, Smith K, Guthridge J, Macwana S, McCarthy S, Sherman M, Daneshfar Hamrah S, Veri M, York K, Walker S, Narpala S, Carroll R, Lin B, Serebryanny L, McDermott A, Barry W, Goldmuntz E, McNamara J, Tedeschi S, Bar-Or A, Khanna D, Clinical Study Team A, James J. Prospective mRNA SARS-CoV-2 Additional Vaccination in Systemic Autoimmune Disease Patients on Immunosuppressive Medications in a Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prospective-mrna-sars-cov-2-additional-vaccination-in-systemic-autoimmune-disease-patients-on-immunosuppressive-medications-in-a-randomized-controlled-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prospective-mrna-sars-cov-2-additional-vaccination-in-systemic-autoimmune-disease-patients-on-immunosuppressive-medications-in-a-randomized-controlled-trial/