Session Information
Date: Saturday, November 6, 2021
Title: Sjögren's Syndrome – Basic & Clinical Science Poster (0296–0322)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Sjogren’s Syndrome (SS) is a systemic autoimmune disease that has considerable impact on health-related quality of life (HRQL). The European League Against Rheumatism (EULAR) Sjogren’s Syndrome Patient Reported Index (ESSPRI) is the most widely used questionnaire to evaluate severity of patient-reported symptoms but is limited in scope. We sought to evaluate predictors of pain, fatigue, and social participation in SS using the Patient Reported Outcome Measurement Information System (PROMIS®) HRQL instruments to supplement ESSPRI findings.
Methods: A cross-sectional evaluation was performed on completed questionnaires of consecutive adult patients during visits to a multidisciplinary Sjogren’s clinic between March 2018-February 2020. Questionnaires included PROMIS short-forms (depression 4a.v1, anxiety 4a.v1, fatigue 8a.v1, physical function 4a.v2, pain interference 8a.v1 (PI), sleep disturbance 4a.v1, participation in social roles and activities 8a.v1) and the ESSPRI. Patients were either classified as SS by 2016 ACR/EULAR criteria or labeled as “sicca not otherwise specified” (NOS) and used as a comparison group. Descriptive statistics were calculated for disease-related and sociodemographic variables and Pearson correlation was used to evaluate the relationship between subdomains of the ESSPRI and PROMIS. Uni- and multivariable linear regression (MVR) models were used to evaluate predictors of PROMIS PI, fatigue, and social participation after controlling for age, gender, race, ethnicity, and symptom duration.
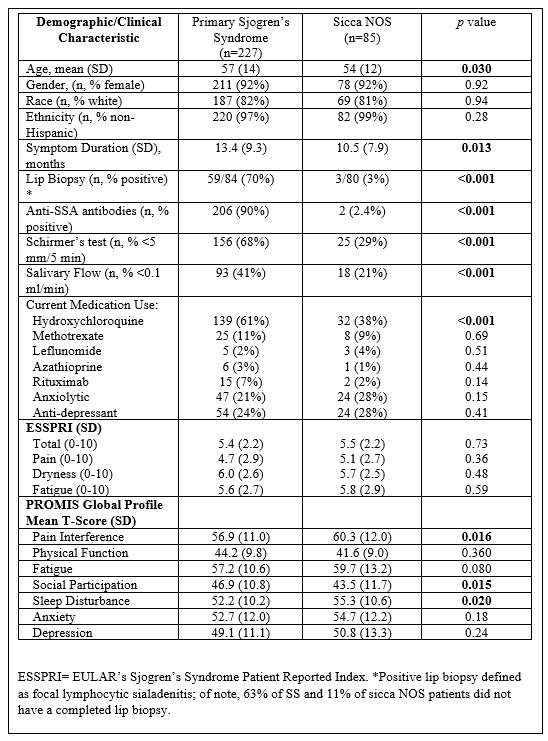
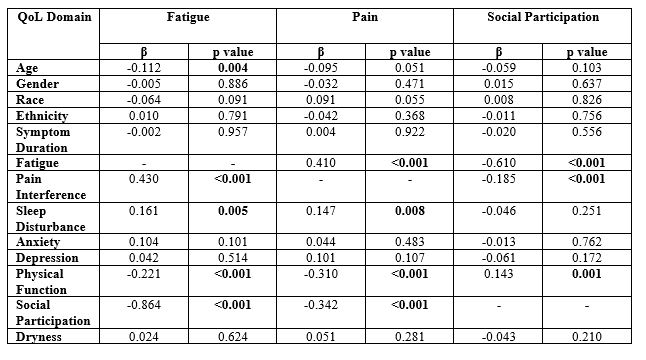
Results: 227 SS patients and 85 patients with sicca NOS were included. Patients with SS were slightly older (mean (SD) years 57 (14) vs 54 (12), p=0.030) and were mostly female and white (Table 1). Mean (SD) PROMIS T-scores for PI (56.9 (11.0)), fatigue (57.2 (10.6)), and physical function (44.2 (9.8)) were at least ½ SD worse than US population normative values. Among SS patients PROMIS PI (r=0.72) and fatigue (r=0.80) highly correlated with respective ESSPRI pain and fatigue sub-domains. PI, sleep disturbance, poor physical function, and impaired social participation predicted fatigue and similarly fatigue, sleep disturbance, poor physical function, and impaired social participation predicted PI (all p’s< 0.01) (Table 2). Fatigue and PI, but not dryness or mood disturbance, were the strongest predictors of social participation in MVR in this SS cohort. Patients with sicca NOS had greater mean T-Scores (SD) for PI (60.3 (12.0)) than the SS cohort in addition to worse social participation (43.5 (11.7)) and SD (55.4 (10.6)) (p’s < 0.05). There were no significant differences in ESSPRI domains between patients with SS and sicca NOS.
Conclusion: In our SS cohort, PROMIS PI and fatigue scores correlated highly with respective ESSPRI domains. Fatigue and PI, but not dryness, were found to be the strongest negative predictors of social participation. Given the ability of PROMIS instruments to identify HRQL domains of high impact on SS patients beyond those ascertained through ESSPRI, these questionnaires should be considered as a supplement when evaluating SS patients in clinical care and trials.
To cite this abstract in AMA style:
DiRenzo D, Robinson S, Bingham C, Baer A, Grader-Beck T. PROMIS Provides a Broad Overview of Health-Related Quality of Life in the Evaluation of Sjogren’s Syndrome [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/promis-provides-a-broad-overview-of-health-related-quality-of-life-in-the-evaluation-of-sjogrens-syndrome/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/promis-provides-a-broad-overview-of-health-related-quality-of-life-in-the-evaluation-of-sjogrens-syndrome/