Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic Sclerosis (SSc) encompasses limited cutaneous (lc)SSc and diffuse cutaneous (dc)SSc, with lcSSc affecting more than 60% of patients and dcSSc associated being burdened by greater morbidity and mortality. Recent expert consensus identified significant clinical events in SSc representing “clinically meaningful worsening” and informing a combined time to event endpoint in the MINIMISE trial (EudraCT: 2019-004139-21). Type I IFN activation correlates with high disease activity and poor outcomes in SSc. This study aimed to evaluate serum Type I IFN’s prognostic value in a “basket” cohort of lcSSc and dcSSc patients using the MINIMISE time to event endpoint.
Methods: A retrospective, longitudinal cohort of patients fulfilling the 2013 ACR/EULAR SSc classification criteria was identified within a national, multicentre observational cohort. The MINIMISE combined Morbi-mortality endpoint was utilized as the clinical outcome in a time-to-event analysis starting from time of serum analysis. IFN score was calculated as previously described (Ross et al 2023)., derived from the serum concentration of CCL2, CCL8, CCL19, CXCL9, CXCL10, and CXCL11 (Myriad RBM). Patients were characterized according to the EUSTAR MEDS dataset. Appropriate statistical tests (Student’s t, Wilcoxon’s, Pearson Chi-squared, and Fisher’s tests) were employed for the analysis. Time to endpoint was analysed using Kaplan-Meier curves, Log-rank test, and Cox Proportional Hazard regression models.
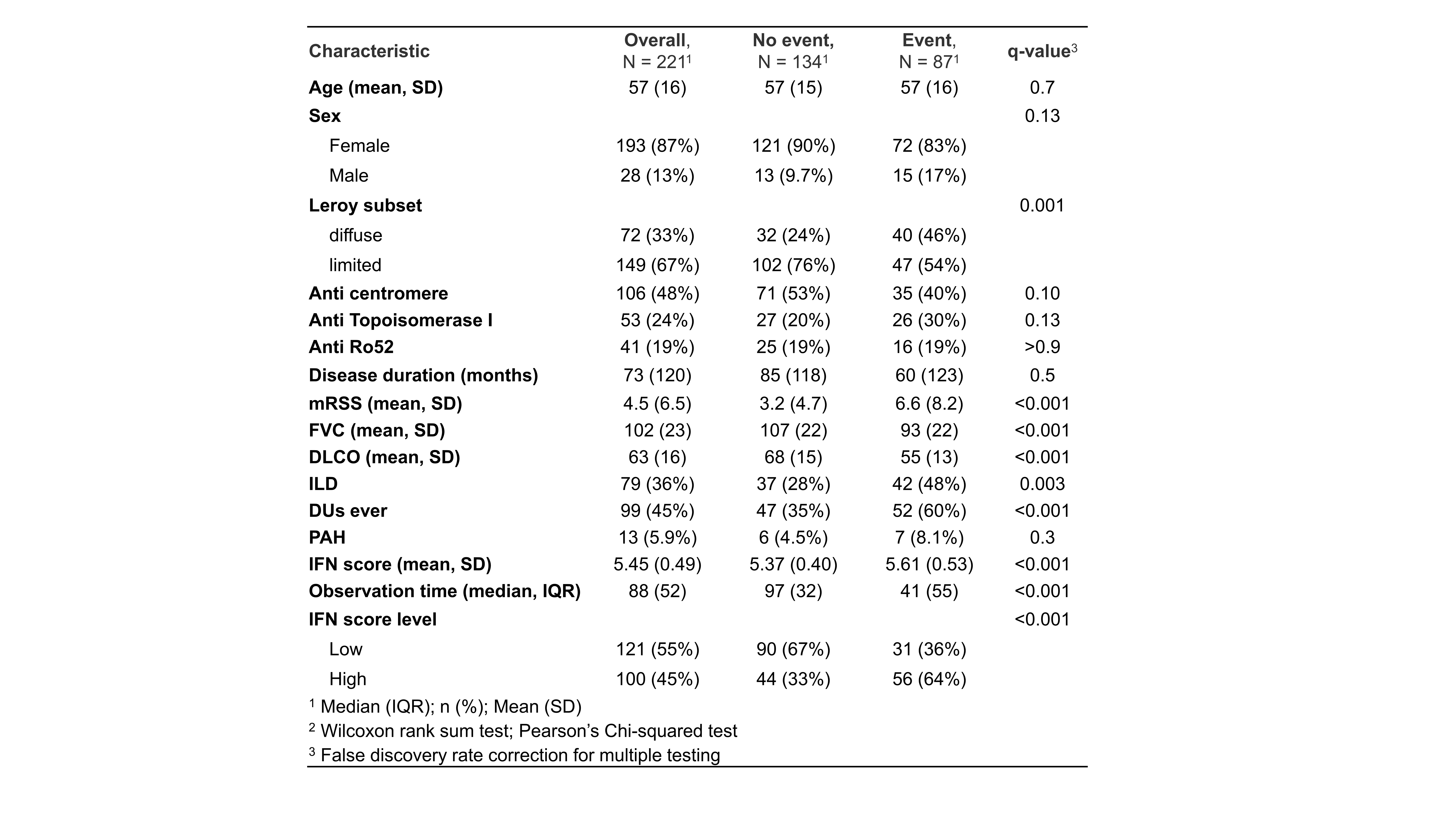
Results: A total of 221 SSc patients (67% lcSSc and 33% dcSSc) were included in the analysis (87% female, mean [SD] age 57 [16] years). The median (IQR) disease duration was 6 (10) years. Median (IQR) follow up was 88 (52) months. 100 (45%) patients had a high IFN score (33 dcSSC, 67 lcSSC) above upper limit of normal in Healthy Controls [HC]), while 121 (55%) were within the HC range (“IFN low”). A significantly higher percentage of patients with a high IFN score met the combined endpoint during follow-up compared to those with low IFN (56% vs 26%, p< 0.001). IFN Low patients had a higher event-free survival (Log rank Chi-Sq=22, df=1, p< 0.001). ILD, mRSS, DU, and diffuse subset were other baseline variables predicting outcomes over time, independently of IFN score. Cox Proportional Hazard models indicated that a high IFN score was associated with a Hazard Ratio (HR) of 2.57 compared to IFN low (95% C.I. 1.64 – 4.03, p< 0.001). When used as a continuous variable, the IFN score maintained an HR of 1.60 (95% C.I.1.18 – 2.16, p=0.002), independent of other clinical variables.
Conclusion: Serum assessment of Type I IFN activity is a valuable prognostic tool in SSc, providing insights into severe outcomes and aiding in patient stratification for clinical trial design. This approach could enhance enrichment and/or stratification strategies for clinical trials and real-life practice.
To cite this abstract in AMA style:
Di Donato S, Minerba M, De Lorenzis E, Hartley C, Bissell L, Ross R, Del Galdo F. Prognostic Value of Serum Type I Interferon in Predicting Morbi-Mortality Outcomes in Systemic Sclerosis: Insights from the STRIKE Basket Cohort [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/prognostic-value-of-serum-type-i-interferon-in-predicting-morbi-mortality-outcomes-in-systemic-sclerosis-insights-from-the-strike-basket-cohort/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prognostic-value-of-serum-type-i-interferon-in-predicting-morbi-mortality-outcomes-in-systemic-sclerosis-insights-from-the-strike-basket-cohort/